The greatest error in microbiome research is generalizing from experimental animals to humans, without accounting for the fact that the experimental animals literally eat their microbiome in a process known as coprophagy. This word comes from the Greek, meaning “the eating of feces.”
Coprophagy is a characteristic, inborn, daily, completely normal trait of many animals. While there are no hard and fast rules like “all small herbivores eat their own feces,” the animals most likely to practice coprophagy are small- or medium-size mammalian herbivores.
The smaller and the more herbivorous the mammal, the more sophisticated and specialized its practice of coprophagy will be.
One of the best-studied groups of coprophagic animals is the leporids, the family that includes rabbits and hares. Their digestive system has a mechanism to turn out two types of feces, soft and hard. The hard feces consists of low-quality food that is not easily fermented. The soft feces consists of high-quality fermentable substrates. The soft fecal pellet is bound by a membrane consisting of vitamin B12 and mucous proteins. The leporid swallows it whole, without chewing. The membrane protects the bacteria inside from the animal’s extremely acidic stomach, allowing them to lacto-ferment the feces for hours, creating a rich array of highly digestible and assimilable nutrients. The leporid will eat the hard feces during the daylight when it is home away from foraging, and will thoroughly chew it, allowing the least digestible parts of its previous food to be re-digested at least a second time.
The reason the small- and medium-size herbivores dominate this digestive strategy is that increasing body size produces a greater increase in digestive capacity than it does energy requirement. So, small animals have higher energy requirements relative to their digestive capacity than do large animals. Herbivores eat plant foods requiring a lot of digestion, but the small ones have low digestive capacity relative to their energy needs. Hence, the smaller and the more herbivorous the animal, the more they need additional digestive adaptations. Coprophagy is one such adaptation.
Another strategy some small herbivores use is to become highly specialized in eating only the most calorically and nutrient-dense plant foods.
Another analogous strategy used by larger herbivores is the rumen, where coarse plant food is fermented before it enters the stomach.
Humans have a different strategy: technology. Soaking, sprouting, souring, and fermenting grains, nuts, seeds, and legumes are ways to increase the quality and digestibility of plant foods. We extend this to animal foods as well when we make foods like fermented sausage, yogurt, and cheese.
Most animals used for lab experiments eat their own feces. They do it consistently, they do it daily, and they do it even when fed a nutritionally optimized diet.
Many nutritional experiments try to minimize this by using cages where the floor is made of raised wire, so that the feces falls through the wire and the animal cannot pick it up or bend down to eat it.
However, this does not work very well, because these animals have much more direct ways of eating their feces:
Mice take feces directly from their anus with their mouth, and then hold the feces with their paws while eating it.
Young rats do the same, though older rats will get lazy and let it fall to the wire mesh before eating it.
Syrian hamsters sit down and bend their head down to take feces out of their anus with their mouth.
Lab animals eat the most feces when they are young and growing, or when they are pregnant or lactating, consistent with using it as a nutrient-dense food.
That coprophagy contributes directly to the nutritional status of lab animals was first observed by Osborne and Mendel in 1911. Raised wire cages were used to try to control this over the next few decades, but researchers could watch animals take feces directly as it left their anus. In the 1930s and 1940s, numerous cages were developed to try to deal with this that focused on restricting the ability of rodents to move, so that they could neither eat feces directly as it left their body nor wander around the cage licking left-behind fecal matter. While a 1949 paper suggested that preventing coprophagy with one of these cages made rats deficient in biotin and folate, feeding liver and feces did not correct the nutritional deficiencies, which made the experiments difficult to interpret. I suspect restricting the movement of the rats was in and of itself harmful to their health.
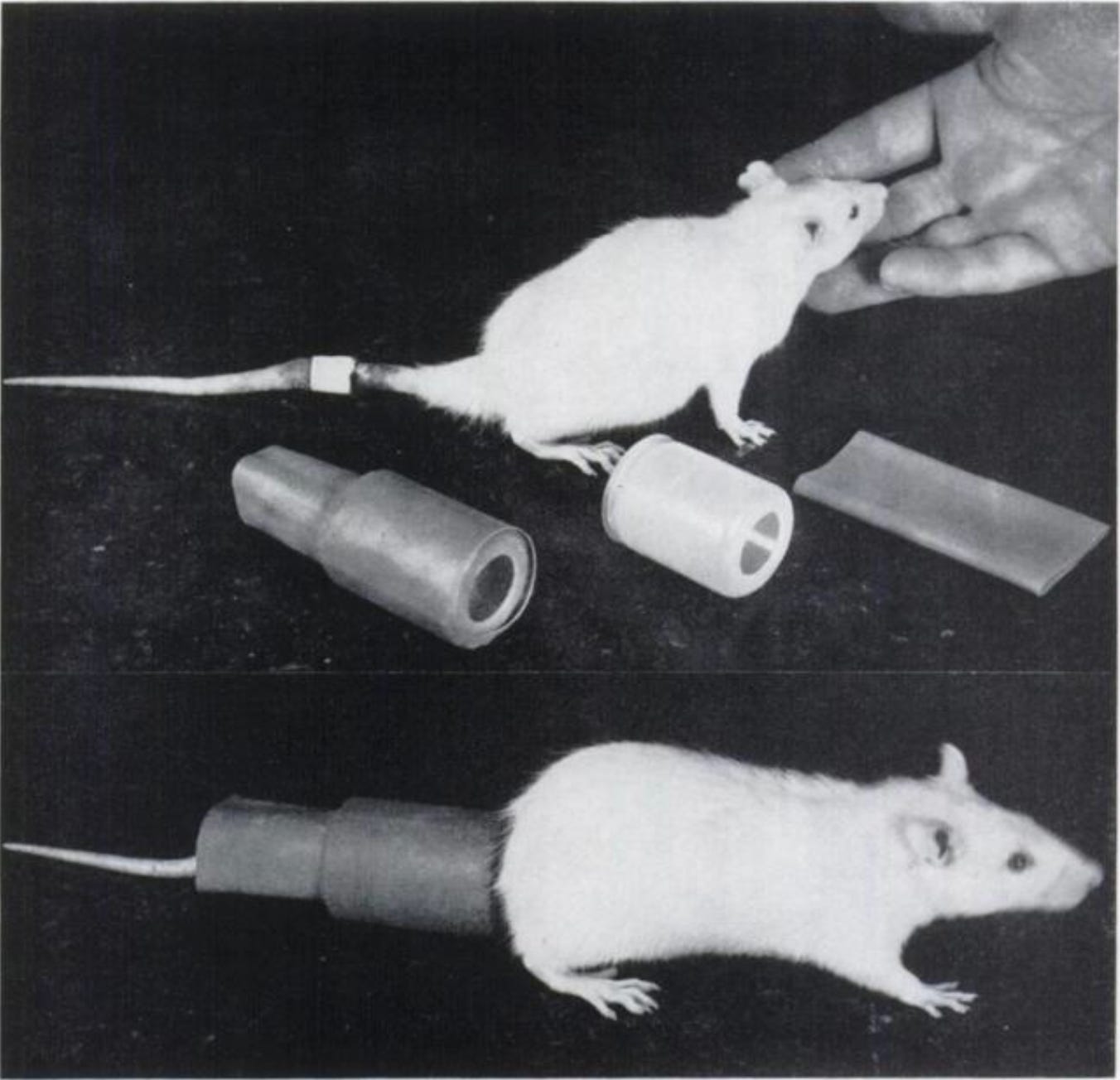
In 1957, Richard Barnes and colleagues at the Cornell Graduate School of Nutrition published a method of coprophagy prevention using a cup that covers the rat’s anus.
This allowed the rat to move normally, while preventing it from accessing any feces and allowing the researchers to collect and measure all the feces.
The Barnes group showed that without these cups, even on raised wire cages, rats still managed to eat 65% of their feces.
They included this wonderful quote at the end, that sums up my major point in this article:
Man’s behavioral development precludes his indulgence in coprophagy with the exception of occasional manifestations of infants and in insane individuals. Therefore, coprophagy marks one of the major differences affecting nutrition in experimental animals and in man. Obviously many experimental results may be subject to erroneous interpretation unless this important nutritional factor is controlled.
Are Vitamins Produced by Gut Microbes Absorbed?
Now let us apply this principle to some practical examples.
Do the vitamins produced by intestinal microbes contribute to our nutritional status?
Clearly it is relevant whether we are eating those microbes for dinner. If we are, then we are subjecting them to the strong acidity of our stomachs and the array of pancreatic enzymes released into our small intestines, and we are using the massive absorptive surface of our small intestine to absorb what is released in the digestive process.
And yet, as humans we do not eat our microbiome for dinner, while lab animals do.
Let’s take our deepest look into biotin.
Does Biotin Produced by the Intestinal Microbiota Get Absorbed?
In 1986, a group of researchers used a device known as a T-cannula to directly infuse biotin into the cecum of minipigs, showing that at least 18% and most likely between 49.7-61.3% was absorbed.
In principle, the cecum is the first part of the colon, so this bypasses the small intestine, and the wavelike motion known as peristalsis should carry the biotin forward into the rest of the colon. Thus, plenty of biotin can be absorbed in the colon.
However, there exists an anti-peristaltic movement and movement across the ileocecal valve, which connects the small intestine to the colon, is reversible. As another group studying this topic in rats with a different technique pointed out the following year, infusing the biotin directly into the cecum does not exclude the possibility that it traveled backwards to be absorbed in the small intestine.
Oddly, the only mention of coprophagy in the 1986 article at all is, “Coprophagy aside, an efficient absorption of the vitamins by the large bowel mucosa is a prerequisite if a supply from the hindgut microflora is postulated.”
Why would we place coprophagy aside? If animals eat their feces, they can absorb whatever is in their feces in the small intestine. We need not invoke absorption in the large intestine.
In 1990, this same group used the same technique to show that avidin infused directly into the cecum of minipigs caused biotin deficiency. Avidin is a protein from uncooked egg white that binds to biotin and prevents its absorption. While they made no effort to prevent coprophagy in their 1986 paper, this time around they “individually housed” the minipigs “in metabolism cages with slatted floors to prevent coprophagy.”
I cannot find any information about coprophagy practices in adult minipigs. However, newborn piglets are known to consume their mother’s feces, which is a nutritionally important source of iron for them. Regardless, the authors evidently considered coprophagy a concern, yet cited no evidence that slatted floors are sufficient to prevent it in minipigs, and reported no attempt to observe whether coprophagy occurred during the course of their experiment.
The avidin reduced the amount of biotin excreted in the urine, and increased the amount that appeared in the feces. When they compared these changes to the amount of biotin minipigs appear to require, they estimated from the urinary biotin that the intestinal microbiota could supply 1.7% of the requirement through direct colonic absorption, and from the fecal biotin that it could supply 17% of the requirement.
These estimates suggest that the microbiota is a minor source of biotin at best.
However, it isn’t clear that this reflects biotin produced by the intestinal microbiota and absorbed directly in the colon. The avidin could move from the cecum into the small intestine and inhibit the absorption of dietary biotin or biotin released into the bile. To the extent coprophagy is a concern in minipigs — as the authors suggested — they provided no evidence that they effectively prevented it.
In 1987, a different group isolated colonic absorption much more effectively in rats. They anesthetized the animals, then operated on their colon to form two loops where food could be passed around in the same segment repeatedly for hours without ever exiting the segment. This left the blood supply and lymph in tact, but prevented biotin infused in one segment from being absorbed in another or from leaving the animals in the feces. Since the animals were anesthetized during the experiments, they wouldn’t be able to eat their feces anyway.
This experiment showed that absorption in the small intestine is nearly complete in 3 hours of circulating through the artificial loop, whereas absorption from the same time circulating in the colon loop is 27%. Thus, the colon of rats can definitely absorb biotin, but its absorptive capacity is less than a third of the small intestine.
Human colonic cells express the sodium-dependent multivitamin transporter (SMVT) on their surface in vitro, so if this experiment had been done in a human, we would likely observe something similar. Indeed, an abstract I cannot find the full text of claims that infusing biotin directly into the transverse colon of humans with colonostomies led to absorption, though it was “better by mouth than from the colon.”
The authors of the rat study concluded, “This theoretical result emphasizes the potential quantitative significance of the distal intestine, where residence times often exceed those of the small intestine, in the biotin economy of the rat.”
In other words, while the colon has less absorptive capacity than the small intestine, food spends more time there, so total absorption from the colon may be quantitatively important.
While it might be, this would only be relevant if the microbiota are producing biotin that they secrete into the lumen of the colon in free form, since biotin from food would probably be completely absorbed in the small intestine before the food arrived in the colon. While certain bacteria found in the intestines do secrete biotin into the culture medium in vitro, it is not clear from such experiments whether and how much biotin makes it into the intestinal lumen without being consumed by other bacteria under natural in vivo conditions.
In fact, it would appear that the Barnes group had settled this in the negative for rats back in 1958.
Four groups of rats were fed biotin-sufficient or biotin-deficient diets either with or without anal cups preventing them from eating their feces. Only the rats on the biotin-deficient diet with the anal cups developed symptoms of biotin deficiency. While they did not demonstrate the classical symptoms of severe deficiency, they developed skin sores where the anal cups were pressing into their skin, their biotin levels dropped, and they did not gain as much bodyweight as the other groups. When extra biotin was given to all the rats at the end of the experiment, they were the only group to experience a rapid increase in bodyweight.
If rats can absorb nutritionally important amounts of biotin produced from their intestinal microbiota directly from their colon, why do they need to eat their feces to prevent deficiency?
On the balance this suggests that the colon expresses biotin transporters so it can mop up whatever may happen to be floating around, yet under normal conditions there isn’t much floating around, so that colonic uptake is generally not very important.
Alternatively, it might suggest that colonic cells nourish themselves with these transporters, but rarely take up enough to be able to nourish the body systemically.
Are Other Nutrients Produced by the Intestinal Microbiota Absorbed?
In this section we cut to the chase. Putting aside all evidence that intestinal microbes produce other nutrients or that colonic transporters for those nutrients exist, let’s go straight to this question: what good does any of this do when you stop an animal from eating its own feces?
The Barnes group produced a series of experiments in the late 1950s showing the following:
Rats fed a diet deficient in B12 have slower growth, but not if they are allowed to eat their own feces.
Essential fatty acid deficiency develops more quickly when rats are prevented from eating their feces. They interpreted this to mean that there are essential fatty acids in the feces, but I think this may have been because B vitamins from the feces (such as biotin, riboflavin, and B6) enable the elongation of linoleic acid to arachidonic acid. However, it is also true that polyunsaturated fats can be present in feces.
Vitamin K-deficient diets cause defective blood clotting in rats only when coprophagy is prevented.
In mice, the Japanese researcher Koichi Ebino showed that B12 and folate deficiency during pregnancy will increase miscarriages and stillbirths, but only when restrained in a way that prevents coprophagy.
All of these experiments raise the question: if these nutrients are produced and absorbed in the colon, why do the rats and mice need to eat their feces to prevent deficiency?
I do not want to give the impression that nutrient deficiencies do not ever occur in these animals as long as they eat their feces. As I described in “They Did the Same Thing to the Lab Rats They Did to Us,” the development of purified rodent diets in the 1970s led to two decades of nutrient deficiencies until these diets were largely optimized in the 1990s. This occurred even though typical experiments do not impose restraints on coprophagy. Most likely the purified diets contain much less fermentable substrate to allow microbial vitamin synthesis than the traditional chow diets, and microbial synthesis can go a long way toward helping rodents meet their nutritional requirements as long as they eat their feces.
Does the Microbiome Cause Obesity?
Now let’s turn to a different topic: does a bad microbiome cause obesity?
The seminal paper published in Nature in 2006 that launched this line of inquiry showed that transplanting the fecal microbiome from obese mice to lean mice made the lean mice become obese.
Matthew Dalby showed that the entire result didn’t even exist: the mice receiving transplants from lean donors had the same ending bodyfat percentage as those receiving transplants from obese donors. The authors took advantage of a statistical artifact known as regression to the mean to claim an effect.
Stephan Guyenet cited this in 2018 as one of the three reasons he did not cover the microbiome angle of obesity in his book, The Hungry Brain.
With that said, let’s imagine for a moment the finding was real.
How likely would it be to generalize to humans?
The authors reported no attempts to prevent coprophagy. What if the substances in the feces that made them fat had to be eaten? This could be as simple as the feces having a higher calorie content!
Even if the mouse experiment had been convincing, what we would need for evidence this works in humans is randomized controlled trials showing that fecal microbiome transplants from lean individuals can reduce adiposity.
A meta-analysis of six trials with a total of 154 patients published in 2020 found low to very low-quality evidence for an impact on metabolic syndrome parameters at 2-6 weeks out, but no effect on obesity parameters at that time point or at 12 weeks out.
This is not to say that there is no role for the microbiome in obesity. It is simply to say that the field has not yet born much fruit and its foundation was never solid to begin with.
Microbiome Research Often Ignores Coprophagy
Even though we have a strong body of literature developed over more than a century showing that coprophagy is a primary nutritional strategy of lab animals, microbiome studies routinely ignore this fact to this day.
We saw above that a paper published in Nature, now with 12,290 citations, made a big splash purporting to show something with potential relevance to humans, yet made no effort whatsoever to heed Barne’s warning from 1957 that “many experimental results may be subject to erroneous interpretation unless this important nutritional factor [coprophagy] is controlled.”
In the “Your Microbiome Will Not Save You” Section of High Protein? You Need More Biotin, I discussed a more recent study on biotin that had this same problem.
In mice broad-spectrum antibiotics, fecal microbiota transplants from severely obese humans, and obesity-producing diets all decreased serum biotin. Conversely, bariatric surgery and prebiotics both increased their biotin status. As I discussed, there are many reasons why obesity would lower biotin status that are not mediated by the microbiome. While it would be fascinating to show that fecal microbiome transplants improve biotin status in humans (which, if it worked, would likely be through improving inflammatory status), showing they can hurt biotin status in mice tells us very little that we can generalize to humans when they made no mention of feces or coprophagy and made no effort to prevent this practice.
The Bottom Line
The bottom line?
Lab animals eat their microbiome.
Any microbiome experiment done in a lab animal with the intention to show proof of principle with potential relevance to humans must take measures to prevent coprophagy.
The way to show an effect in humans is with a randomized controlled trial of a fecal microbiome transplant.
If you see a microbiome paper in lab animals that does not control coprophagy, treat it with deep skepticism and wait for better evidence before surmising the finding may have relevance to humans.
Vitamin absorption in the colon is probably limited to what may offer some nutritional support to the colonic cells and probably plays very little if any role in nutritional support for the rest of the body. The main effects of the microbiome on nutritional status are probably indirect effects mediated by direct effects on intestinal motility and inflammation.
The human equivalent of the small herbivore’s coprophagy is our technological development to grind, soak, sprout, ferment, and cook coarse plant foods, and to expand these technologies to animal foods.
Stay Immune Through the Winter
My new 7-page quick guide on how to not get sick this winter, Staying Immune Through the Winter, is free for everyone. All you need is a free or paid subscription to my Substack.
If the box above says “subscribed,” this means you are a Masterpass member and can download the guide here.
If the box says “upgrade to paid,” then you are a free subscriber and your guide is currently sitting in your inbox. Search your mail, spam, and trash for “staying immune through the winter” and make sure anything from Substack is going to primary.
If it allows you to enter your email address, do so, hit “subscribe,” and then find the email from “Chris Masterjohn, PhD” with the subject “Welcome to My Newsletter!” If you can’t find this, try searching for either of those terms and for “Substack.” At the top of the welcome email you will find your downloadable guide to not getting sick.
Join the Next Live Q&A
Have a question for me? Ask it at the next Q&A! Learn more here.
Join the Masterpass
Masterpass members get access to premium content (preview the premium posts here), all my ebook guides for free (see the collection of ebook guides here), monthly live Q&A sessions (see when the next session is here), all my courses for free (see the collection here), and exclusive access to massive discounts (see the specific discounts available by clicking here). Upgrade your subscription to include Masterpass membership with this button:
Learn more about the Masterpass here.
Please Show this Post Some Love
Please like and share this post on the Substack web site. This will help spread it far and wide, as subscriber likes drive the Substack algorithm. Likes from paid subscribers count the most, but likes from free subscribers come in the highest numbers and are the foundation for spreading the content.
Take a Look at the Store
At no extra cost to you, please consider buying products from one of my popular affiliates using these links: Paleovalley, Magic Spoon breakfast cereal, LMNT, Seeking Health, Ancestral Supplements, MASA chips. Find more affiliates here.
For $2.99, you can purchase The Vitamins and Minerals 101 Cliff Notes, a bullet point summary of all the most important things I’ve learned in over 15 years of studying nutrition science.
For $10, you can purchase The Food and Supplement Guide for the Coronavirus, my protocol for prevention and for what to do if you get sick.
For $10, you can purchase Healing From COVID Vaccine Side Effects for yourself or a loved one if dealing with this issue. It also contains an extensive well-referenced scientific review, so you can also use this just to learn more about my research into the COVID vaccines.
For $29.99, you can purchase a copy of my ebook, Testing Nutritional Status: The Ultimate Cheat Sheet, my complete system for managing your nutritional status using dietary analysis, a survey of just under 200 signs and symptoms, and a comprehensive guide to proper interpretation of labwork.


I wasn't quite ready for this, I must say.
Really appreciate the rigorous research and time taken to explain to laypeople like myself.