Disclaimer: This is not medical or nutritional advice. Please take this as education meant for personal empowerment, and make your own decisions with, where valuable, the help of a caring health care provider.
Yesterday, I held a live Q&A with Masterpass members, and one of the questions that came up was whether we should be concerned about alpha-lipoic acid supplements hurting methylation as described in this rat study from 2010.
After searching among the papers citing this study, the Examine.Com human effect matrix for lipoic acid, and the pubmed results for “lipoic acid randomized homocysteine,” I believe this is the only paper showing lipoic acid hurts methylation.
So let’s dig into it.
Lipoic Acid Metabolism and Jargon
As shown in Figure 1a, lipoic acid has two sulfur atoms, labeled “S.” In its oxidized state, they are bound together in a disulfide bond. It its reduced state, they are separated into two sulfhydryl groups, also known as thiol groups. This reduced form is known as dihydrolipoic acid because there are two extra hydrogen atoms added to each sulfur atom.
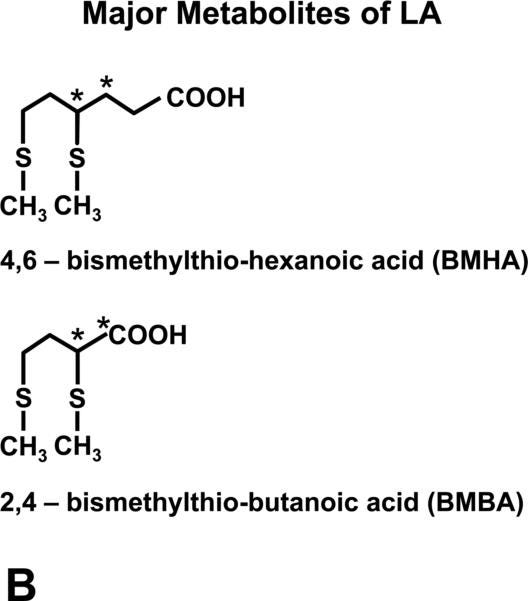
Figure 1b shows that lipoic acid is mainly metabolized into two forms that have methyl groups added to the thiol groups, making methylthiol groups.
If you count the points of the zig-zagging line at the top of the molecules, where each point represents a carbon atom, you will see that the eight carbons of lipoic acid are shortened to either six or four carbons. Lipoic acid is called an acid because the “COOH” or “carboxyl” group is acidic. Specifically, it is a carboxylic acid.“Bis” refers to two things that are not connected to one another. “Hexanoic” means a 6-carbon carboxylic acid. “Butanoic” means a 4-carbon carboxylic acid. Hence, these two metabolites are bismethylthio-hexanoic acid (a 6-carbon carboxylic acid with two separate methylthiol groups) and bismethylthio-butanoic acid (a 4-carbon carboxylic acid with two separate methylthiol groups). The numbers simply refer to counting the carbons from right to left and numbering where the methylthiol group are.
The “alpha” in alpha-lipoic acid refers to the fact that the first carbon with a functional group (that is, something interesting popping off the molecule) has the acidic carbon. This is a bit circular. The first functional group is the COOH and it is on carbon 1, so carbon 1 is the alpha carbon, and the alpha carbon has the COOH, so it an alpha-carboxylic acid. Thus the “alpha” is sort of redundant and often dropped off the name.
Excess Lipoic Acid is Methylated
Putting all the jargon aside, the point is this:
—> Each lipoic acid molecule that does not get incorporated as an enzymatic cofactor is likely to soak up two methyl groups in the course of its removal from the body.
Searching pubmed for these metabolites reveals that, in humans, a single 600 mg dose of alpha-lipoic acid generates far more of the 6-carbon methylated metabolite than anything else, with the 4-carbon metabolite being second, and unmethylated lipoic acid being third. Quite behind these is an 8-carbon methylated metabolite. About 20% of the dose was excreted in the urine within the first 24 hours.
I don’t think we can say for sure how much of the lipoic acid was methylated, because we don’t know how much was quickly stored in the liver or elsewhere for incorporation into enzymes.
However, we can verify that, in humans, the overwhelming proportion of what circulates in the plasma or urine after a typical oral dose is methylated.
That means that, if rats also mainly produce these metabolites, then the principles learned from the rat study about the significance of these metabolites is likely to have some meaningful translation to humans.
In Rats, Lipoic Acid Massively Depletes Methyl Groups
So, let’s return to the rat study.
The animals were injected with 100 mg alpha-lipoic acid per kilogram bodyweight. 30 minutes later, they were killed under general anesthesia and their tissues were analyzed.
The alpha-lipoic acid was a racemic mixture, which means it was half the “R” form — which is found naturally and usable — and half the “S” form — which is a biologically useless byproduct of synthesizing the “R” form. That is, they were injected with the “cheap crap” version of alpha-lipoic acid rather than the fancy “R” form that mimics what you would get from food or synthesize within your own body.
If lipoic acid is methylated, we would expect the following to be involved:
Methionine (from dietary protein, or regenerated from homocysteine using folate and B12, or using betaine (trimethylglycine) is activated by magnesium and ATP to S-adenosyl-methionine (SAM).
The SAM methylates lipoic acid.
In the process, it becomes S-adenosyl-homocysteine (SAH).
SAH is then hydrolyzed (broken down with water) to homocysteine.
The homocysteine either accumulates as itself, is recycled to methionine, or is broken down to cystathionine and further broken down into cysteine via the trans-sulfuration pathway. The cysteine can then be used to make glutathione, taurine, sulfate, or hydrogen sulfide gas (used for dilation of blood vessels or for function of the placenta, for example), depending on the needs of the cell.
Consistent with this, injection of the lipoic acid whacked the liver’s SAM levels by just under 80%!
The SAH in the plasma increased 10-20-fold, and the homocysteine doubled.
One remarkable point here is that the change in blood levels of homocysteine are only 2x, while the depletion of SAM in the liver is 5x and the accumulation of SAH in the blood is 10x-20x. This suggests the following:
—>Changes in blood levels of homocysteine dramatically underestimate the changes in methylation status that they may be reflecting. The direction is reliable, but the magnitude is, at least sometimes, a gross underestimation of the change.
The trans-sulfuration pathway was modestly elevated, as shown by two markers, cystathionine and alpha-aminobutyrate.
Blood levels of cysteine dropped by two-thirds. It is not obvious to me why this would happen, but I suspect it might be a reaction to keep the total sulfur content of the blood relatively constant. Since the liver had a massive amount of SAH and homocysteine it needed to get rid of, it threw those out into the blood instead of cysteine.
Alternatively, the cysteine may have been taken up from the blood into cells of other organs if they needed it to make glutathione to defend themselves against oxidative stress caused by the massive increase in circulating SAH and homocysteine.
Are the Doses Relevant to Humans?
These authors claim that their dose is the bodyweight-adjusted equivalent of 4-10 times the usual human doses of 600-1800 milligrams per day (mg/d). However, they neglected to divide the rat dose by 6.2 to account for differences in surface area. So their dose is actually the equivalent of a human taking 1129 mg/d.1
However, they also neglected to emphasize the obvious elephant in the room: they injected the rats with this dose, and humans most often take lipoic acid orally.
So it’s actually the equivalent of a human receiving 1129 mg/d intravenously, or otherwise injected.
Is the R-Form Worse for Methylation?
They cite a study showing that R-alpha-lipoic (R-ALA) acid has better bioavailability in humans than the racemic mixture they used, and they argue that the R form “might be expected to have more effect on methylation status.”
This strikes me as completely dubious.
First of all, anything injected has 100% absorption, so if the R form had better intestinal absorption than the racemic mixture, this would mean the oral forms have less than 100% absorption and the R form orally might better approach the effect observed in the study where the absorption had to be 100% because it was injected.
Second, the study they cite was using a sodium salt of R-ALA to show it had much better absorption than free R-ALA, which itself has lower absorption compared to the racemic mixture. So whether R-ALA has better absorption, by these measures, depends on whether it is a sodium salt or free-form.
Third, the absorption into plasma does not tell you anything about the fraction quickly taken up by the liver to use as an enzymatic cofactor.
It is well established that R-ALA is the only biologically usable form of ALA, so its “low absorption” in free form compared to the racemic mixture might actually represent superior uptake by the liver for use as an enzymatic cofactor. The sodium salt might much better absorbed, allowing greater liver uptake as well as greater plasma levels.
My suspicion is that what is methylated represents the excess over what is used as an enzymatic cofactor. Therefore, R-ALA would be better used as an enzymatic cofactor and less likely to be methylated than the racemic mixture.
Does Lipoic Acid Increase Homocysteine in Humans?
In a randomized crossover trial of 20 adults with β-thalassemia major, a blood disorder, homocysteine was 11% higher (11.72 vs 10.52) after 8 weeks of taking 600 mg/d alpha-lipoic acid than after 8 weeks of placebo, but it wasn’t statistically significant. They don’t say anything about the formulation, so it was probably the racemic mixture.
In a randomized controlled trial of 42 Korean patients with Parkinson’s disease, the group that took 600 mg alpha-lipoic acid twice a day for a year had their homocysteine levels go up 19.4%, while those in the control group had theirs go up only 9.1%. However, they don’t report the ending values in each group, which is the appropriate variable to compare, and the difference in percent change wasn’t statistically significant. The formulation was Lipo A-HR, made by the Korean company Dalim Biotech. I can’t figure out if it is the R-form or the racemic mixture, so it is probably the racemic mixture.
As far as I know, these are the only studies that looked at this question. They are consistent with a possible small effect on average that these studies were too small to make statistically significant.
However, I think it’s important to note that the most sensitive response to declining methyl groups will always be decreased creatine synthesis. So lower strength, declining muscle mass over the course of months, or depression (the most common symptoms you’d expect from declining creatine status) could be more sensitive than increased homocysteine.
It is also important to note that lipoic acid may increase homocysteine in some susceptible people without doing so for the population average.
That said, it is quite clear that 1200 mg orally over eight weeks or one year does not double homocysteine the way the equivalent does in rats when injected a single time.
Still, if we wanted to compare apples to apples, we would really want to look at the homocysteine levels 30 minutes after the time at which the oral doses maximize plasma concentrations (about 15-20 minutes for the sodium salt of R-ALA, so 45-55 minutes after the dose). Studies like this typically look at fasting values. Most often in the real world we do the same.
Ultimately, this remains an open question.
What to Do About This
Since we know that 600 mg orally in humans generates these same methylated metabolites, and since the equivalent of the dosing here is 1129 mg injected into a 70-kilogram human, it is quite safe to assume that injection of 1100-1200 mg of the racemic mixture of R- and S- alpha-lipoic acid in humans is going to substantially sap methyl groups.
My suspicion is that using a sodium salt of R-ALA orally and spreading the dose evenly across meals rather than taking it all at once are the best ways to maximize absorption while minimizing the amount that is methylated.
In Testing Nutritional Status: The Ultimate Cheat Sheet, I define the following as the most common signs and symptoms of undermethylation:
Fatty liver disease, neural tube birth defects, elevated homocysteine and associated cardiovascular risk, fatigue, poor exercise capacity, histamine intolerance, difficulty ignoring negative thoughts and thought patterns, depression, anxiety, obsessive compulsive disorder, histamine intolerance, inability to adequately eliminate arsenic, inability to properly utilize selenium or excrete excess selenium. Severe deficiencies in methylation could contribute to deficiencies of zinc, copper, and perhaps other positively charged minerals. As with excessive methylation, possibly cancer.
If you suffer from any of these and it correlates with lipoic acid use, it makes sense to manage your lipoic acid use with attention to methylation status.
That means:
Use the R-form and spread the dose out evenly across meals.
Don’t use more than you can clearly justify with a measurable benefit.
Use it on a background diet rich in animal protein, especially eggs and/or dairy, for extra methionine, and in eggs and/or liver for choline (see alternatives here), and in liver, legumes, and leafy greens for folate.
Pair your lipoic acid with an equal dose of trimethylglycine (TMG).2
Make sure you are not deficient in any nutrients needed to support methylation. The Cheat Sheet has a section devoted to this.
If you don’t suffer from any of these things, you probably don’t have to worry about it.
Not sure? Check your homocysteine. If your homocysteine is substantially higher than the 7-9 range, and you are using lipoic acid, the lipoic acid could be contributing. Try the tips above to see if they help bring down the homocysteine.
The Bottom Line
Alpha-lipoic acid saps methyl groups.
Most likely, it is the excess of what can be incorporated into enzymes that is treated this way.
Therefore, using the R-form for superior incorporation into enzymes, and spreading it out across meals to allow for the time needed for that incorporation, is likely to reduce the methylation burden.
If you are taking lipoic acid, your homocysteine is great, and you have no signs or symptoms of undermethylation, you probably don’t need to worry about this.
However, if you are taking lipoic acid, and you do have signs or symptoms of undermethylation, or you do have high homocysteine, then the lipoic acid might be part of the problem. Follow the tips above to see if they help.
If you are not taking lipoic acid and are about to start, keep these principles in mind. Make sure your diet will support robust methylation and keep a lookout for any signs of undermethylation along the way.
Please Show This Post Some Love
Let me know what you think in the comments! And please like the post if you found it valuable, and share the post with others if you believe they too would find it valuable.
Join the Next Live Q&A
Have a question for me? Ask it at the next Q&A! Learn more here.
Subscribe
Subscribe or upgrade your subscription here:
Join the Masterpass
Masterpass members get access to premium content (preview the premium posts here), all my ebook guides for free (see the collection of ebook guides here), monthly live Q&A sessions (see when the next session is here), all my courses for free (see the collection here), and exclusive access to massive discounts (see the specific discounts available by clicking here). Upgrade your subscription to include Masterpass membership with this button:
Learn more about the Masterpass here.
Take a Look at the Store
At no extra cost to you, please consider buying products from one of my popular affiliates using these links: Paleovalley, Magic Spoon breakfast cereal, LMNT, Seeking Health, Ancestral Supplements. Find more affiliates here.
For $2.99, you can purchase The Vitamins and Minerals 101 Cliff Notes, a bullet point summary of all the most important things I’ve learned in over 15 years of studying nutrition science.
For $10, you can purchase The Food and Supplement Guide for the Coronavirus, my protocol for prevention and for what to do if you get sick.
For $29.99, you can purchase a copy of my ebook, Testing Nutritional Status: The Ultimate Cheat Sheet, my complete system for managing your nutritional status using dietary analysis, a survey of just under 200 signs and symptoms, and a comprehensive guide to proper interpretation of labwork.
100 mg/kg divided by 6.2 for converting from rats to humans multiplied by 70 kg for the “standard reference man.”
We want roughly two molecules of TMG for each molecule of lipoic acid, since each TMG molecule donates one methyl group and each excess lipoic acid molecule absorbs two. The molar mass of TMG is roughly half of the molar mass of lipoic acid, so dosing them equally approximates this.



Interesting, thanks for posting this summary and review. Would be fascinating to hear a video/podcast conversation between you and Dr. Burt Berkson talking about ALA and how it functions, clinical use, etc. He has treated patients for years with it, conditions related to liver disease, cancer treatment, autoimmune, and some others, and he has some remarkable published case studies. I heard he advises b complex injections after ALA infusions due to it depleting their levels in some way, but I couldn’t find a citation for that claim to know the mechanism of how that happens.
So fascinating - thank you. I just listed to your podcast on niacin as well and deeply appreciate your insight on how these two supplements can have a huge negative impact on methylation.