Can Biotin Help Detoxify Oxalate?
This is a hypothesis that may reconcile some anecdotes and has many hints suggested in the enzymological literature.
Disclaimer: I am not a medical doctor and this is not medical advice.
Sally Norton in her new book Toxic Superfoods: How Oxalate is Making You Sick brought it to my attention that oxalate inhibits several enzymes, including the biotin-dependent enzyme pyruvate carboxylase.
In this post, I will go a step further: I will propose the hypothesis that the biotin-dependent enzyme pyruvate carboxylase actually detoxifies oxalate.
But back to the story.
This point Norton raised struck me as very relevant to my own investigation of what I believe is a genetically driven state of biotin deficiency that is likely a compound heterozygous mix of biotinidase deficiency and deficiency of a biotin transporter, or, based on more recent labwork possibly a mix of deficiencies in the biotin-dependent enzymes pyruvate carboxylase and propionyl CoA carboxylase. I should soon have a wealth of genetic data to offer final clarity on this.
One reason it struck me as very relevant is that I know for a fact I have — or at least have had — issues with oxalate. Most glaringly, roughly two decades ago I discovered how amazing sweet potato fries were, so bought a bunch of sweet potatoes. I ate some of them as fries, and some baked, averaging one to two sweet potatoes per day. By the end of the week, I had a limp. My emerging neck tension had also increased considerably.
“I must be eating something high in oxalate!” I said to myself.
I looked it up. Sure enough, sweet potatoes were up there with spinach. I cut them out. Within a week, the limp was gone.
Could I be confusing oxalate toxicity with biotin deficiency? Not entirely, since my concern about biotin is that my biotin levels are discrepantly low compared to my diet.
Could biotin deficiency make me more vulnerable to oxalate? This would explain why my mother has a need for high-dose biotin for her hair health and is vulnerable to oxalate kidney stones, which I think reflects a gene we inherited from my maternal grandfather, who had severe hearing loss rather early in life.
This also seemed very relevant to numerous discussions brought up in the comments on my biotin posts.
In the comments on When High-Dose Biotin Is Truly Needed, JenP wrote that two of her children were supplementing with biotin to help with yeast and oxalate intolerance. They did well on 5 milligrams, but 10 milligrams made their hands break out in terrible rashes that took months to heal.
Similarly, KattyGirlYo asked me on Twitter why 2 milligrams of biotin gives her “excess keratinization of the skin.”
Could these represent what Sally Owens calls “oxalate dumping”?
Norton singles biotin out as one of three B vitamins of special concern, since oxalate hurts biotin-dependent enzymes. In Table 15.6, she lists biotin in doses ranging from 0.5 to 10 milligrams per day to help “reduce dumping side effects, including brain symptoms.”
I am guessing this comes from Owens’ work. On her web site she notes that oxalate produces symptoms similar to biotinidsase deficiency, which is a genetic deficiency in the ability to recycle biotin. Since one of these is runaway yeast infections, she suggests using “a supplement that furnishes biotin in [milligrams] rather than [micrograms]” to help with chronic yeast issues. She also lists “strange rashes” as a sign of “dumping.”
Norton prefers the word “clearance” to “dumping,” but embraces the idea that low-oxalate diets can reduce circulating oxalate down to a threshold that favors oxalate clearance over oxalate accumulation, and can lead to such symptoms. These can include any symptoms of oxalate toxicity, and she lists eczema as one in Table 10.1. In the beginning of chapter 9, she lists among the symptoms of “clearance,” gritty snot, fine grit, white crystals, or white dust coming out of the skin and nail beds, skin peeling off the fingers and toes, red or white bumps on the skin, and terrible rashes.
I am generally skeptical of “detox reactions.” I believe they exist, but I also believe they are often used to set up unfalsifiable defenses of protocols that do not work. However, it makes complete sense to me that reducing circulating oxalate beneath a certain threshold will stimulate the clearance of accumulated crystals. Formation of crystals would be a defense against biochemical toxicity, but at the expense of damaging connective tissues and mucous membranes. Reversing this process could temporarily increase biochemical toxicity. Moreover, when crystals break up they never fully solubilize automatically, so the breaking up of crystals could cause physical irritation of tissues.
Neither Owens (to my knowledge) nor Norton suggest that biotin could cause clearance reactions.
Yet JenP and KattyGirlYo report experiences that could be interpreted in that light. JenP was particularly confused by the reaction:
I was told later that it probably pushed out oxalate too fast for their bodies to handle, thus the rash. This has never totally made sense to me, as biotin is meant to help the body eliminate oxalates, and should be correcting processes that oxalate has disrupted.
Evidently someone is saying biotin can promote clearance of oxalates.
Can it?
This is only a hypothesis, but as I looked into the enzymological literature, I became more and more convinced that the biotin-dependent enzyme pyruvate carboxylase is a plausible candidate for a means of detoxifying oxalate to formate, which can be utilized in methylation or further broken down to carbon dioxide.
First, let’s take a look at what oxalate is.
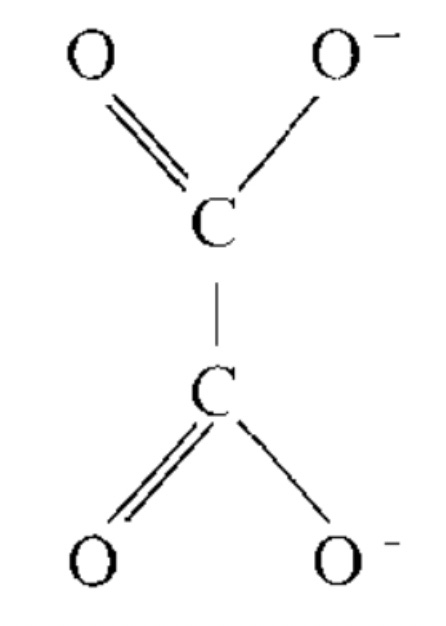
Oxalate is Two Carbon Dioxide Molecules Bound Together
This is the structure of oxalate:
It is the simplest dicarboxylic acid. Two carboxyl groups.
What is a carboxyl group? It is a molecule of carbon dioxide that has been bound to carbon-based, organic molecule.
Oxalate is an organic molecule because it has two carbons.
But both carbons belong to a carboxyl group.
So what is it?
It is an organic molecule formed by gluing two molecules of CO2 together.
And THIS is our formidable enemy, against whom we have no defense except to sequester as crystals that destroy our tissues?
This thing is in any diet on earth at tens or hundreds of milligrams per day, has toxic effects at physiological concentrations, and we can’t split it into two molecules of CO2?
The only way we can break this thing down is to hope our gut bacteria contain the rare organisms that specialize in feeding off of it?
Something just doesn’t add up. There should be some way we can break this thing apart into two molecules of CO2 and exhale it.
Before we make some specific wild hypothesis supporting this, let’s take a look at how bacteria get rid of oxalate, since everyone seems to agree that some bacteria can do this.
Bacterial Decarboxylation of Oxalate
This is not comprehensive, just illustrative.
Several Pseudomonas species metabolize oxalate. Pseudomonas oxalaticus uses succinyl CoA to donate the CoA to oxalate, forming oxalyl CoA. It then reduces oxalyl CoA to glyoxylate and CoA, using NADPH. Thus, pantothenic acid or vitamin B5 is used in the form of CoA, and niacin or vitamin B3 is used in the form of NADPH.
To use oxalate for anabolic reactions, the bacterium feeds glyoxylate into the glycerate pathway. To catabolize oxalate for energy, oxalyl CoA is decarboxylated to formyl CoA and CO2. The CoA that is released is transferred to a new oxalate molecule, while NAD+ is used to oxidize formate to CO2.
There are several pink-pigmented Pseudomonas organisms (AM1, AM2, ruber, and extorquens) that do the same thing, but have an alternative way of using oxalate for anabolic reactions: they make serine react with glyoxylate to form hydroxypyruvate and glycine, while folate donates a carbon to glycine to regenerate serine.
The main point here is that they all use the enzyme oxalyl CoA decarboxylase to break off the first CO2, making formate, and then use formate dehydrogenase to release the last CO2.
Formate is simply CO2 with a hydrogen sticking off of it:
So, if bacteria want to destroy oxalate, they need to oxidize it and rip apart the two CO2 to be released.
Human Formate Metabolism
Now let’s look at something else that is well accepted, this time in humans: formate metabolism.
Formate can be formed from serine, glycine, methionine, choline, and methanol, and may also be formed during synthesis of cholesterol and other sterols and the breakdown of tryptophan. Gut bacteria can make formate and methanol, and exposure to formaldehyde or methanol in industry or laboratory work are additional sources.
An overflow of formate is a major cause of methanol toxicity, but we have excellent ways of putting formate to good use or detoxifying it when we are not exposed to excessive or surprise exogenous doses.
Since formate possesses one carbon, it can provide the one carbon for “one carbon metabolism,” also known as methylation. The principle disposal route for formate is to provide the carbon that becomes the methyl group of methylfolate.
Nevertheless, if we add more formate to folate than is needed for methylation, we use the niacin-dependent enzymes aldehyde dehydrogenase 1 and 2 to break the formate off of folate in the form of CO2, releasing it while we exhale.
So, one thing is definitely true: like bacteria, we can use niacin to oxidize formate to CO2.
Thus, we clearly possess the second expected step of oxalate detoxification.
Now, do we have a way to decarboxylate oxalate to form formate in the first place?
That is, do we also possess the means to engage in the first step of oxalate detoxification?
This is where it gets controversial.
Pyruvate Carboxylase is a “Multi-Substrate Enzyme”
The textbook function for pyruvate carboxylase is to allow glucose to enter the citric acid cycle using itself as its own fuel, and to allow protein or lactate to be used to synthesize glucose. Glucose entering the citric acid cycle is needed for it to be burned for energy, and is also needed for it to be converted to fat. As a result of this, pyruvate carboxylase is needed indirectly to synthesize anything fatty, such as the elongated forms of the essential fatty acids, the myelin sheath of neurons, or lipoic acid, which is a modified fatty acid that acts as a cofactor for several enzymes.
Specifically, pyruvate carboxylase does all these things by taking CO2 that has been dissolved in water to form bicarbonate, and adding it to pyruvate to form oxaloacetate.
The biochemical adage, “fat burns in the flame of carbohydrate” refers to the fact that fatty acids generate the two-carbon acetyl CoA, but cannot lead to the net synthesis of the four-carbon oxaloacetate. As a result, the acetyl CoA they release cannot be fully burned for energy and condenses with itself to form ketone bodies, some of which leave in the urine. However, in the presence of glucose, pyruvate carboxylase can convert pyruvate, derived from glucose, to oxaloacetate, and allow the acetyl CoA to be burned for energy.
During a ketogenic diet, as an example, or fasting, oxaloacetate is used in the liver for gluconeogenesis, and incoming pyruvate is in short supply. This depletes the liver of oxaloacetate. Acetyl CoA from fatty acid oxidation accumulates. There is no oxaloacetate to allow the acetyl CoA to enter the citric acid cycle, so the acetyl CoA molecules condense with each other to form ketones.
Glucose taken up by the brain, muscle, and fat, is used to make pyruvate, and then pyruvate carboxylase converts the pyruvate to oxaloacetate, and this allows the ketone bodies made by the liver to be used for energy in those tissues.
However, protein can enter the citric acid cycle by other means to produce oxaloacetate, and this is why, in High Protein? You Need More Biotin, I suggested that one sign of biotin deficiency would be hyperglycemia that is mitigated by dietary protein. The adage should be “fat burns in the flame of oxaloacetate.”
Despite this singular textbook explanation for the enzyme, in vitro — in a lab dish — the enzyme can easily run backwards to decarboxylate oxaloacetate. As one group wrote in 1974, “pyruvate carboxylase is a multi-substrate, multi-effector enzyme.”
In vivo, in the live human or animal, pyruvate carboxylase does not run backwards, but this is only because the concentration of oxaloacetate is always vanishingly small. This is why urinary organic acid tests do not even bother measuring it. Any oxaloacetate that is not used to fuel the citric acid cycle or be converted to aspartate using vitamin B6 will be rapidly converted to malate, which is the “backwards reaction” of the last step of the citric acid cycle.
Even earlier, in 1963, researchers had shown that pyruvate carboxylase can carboxylate other molecules. Specifically, it carboxylated alpha-ketobutyrate — a product of cysteine metabolism — at 3% of the rate at which it carboxylated pyruvate.
Thus, pyruvate carboxylase is at least capable of running backwards to perform decarboxylation and of metabolizing substrates other than pyruvate and oxaloacetate.
Oxalate “Inhibits” Pyruvate Carboxylase
The interest in the ability of oxalate to “inhibit” pyruvate carboxylase goes back at least to the 1960s, when numerous authors showed that oxalate inhibits this enzyme in baker’s yeast, bacteria, and rat liver.
In the 1980s, research picked up on the physiological relevance of this inhibition.
In chicks, oxalate was shown to inhibit gluconeogenesis, and also to inhibit cholesterol synthesis and the conversion of lactate into fatty acids.
As noted in a paper from 1983, oxalate is present in human plasma at concentrations between 20 to 40 micromoles per liter. At these concentrations, experiments suggest it should freely enter liver and muscle cells where it would inhibit pyruvate carboxylase along with at least two other enzymes of pyruvate metabolism.
This paper showed that in rat liver cells, physiologically relevant concentrations of oxalate inhibit citric acid cycle activity by a whopping 48%.
As would be expected, this leads to reduction in the amount of mitochondrial NADH (the opposite of what a respiratory chain disorder would do), and to increased ketogenesis (as a result of decreased oxaloacetate).
The reason I put “inhibits” in quotation marks is that not one of these studies — indeed, no study I can find anywhere — looked at whether oxalate was being decarboxylated to formate while it was “inhibiting” pyruvate carboxylase.
In other words, it is inhibiting the reaction you want to happen, but is it only staying stuck in the enzyme and blocking the pathway, or is it using the enzyme for the first expected step in its detoxification, its decarboxylation to formate?
Can Pyruvate Carboxylase Convert Oxalate to Formate?
Using pyruvate carboxylase isolated from chicken liver mitochondria, one group showed in 1966 that oxalate inhibited the forward reaction in a non-competitive manner, but inhibited the backward reaction — the decarboxylation of oxaloacetate — in a competitive manner.
What that means is that oxalate was binding somewhere in the enzyme other than where pyruvate binds, but in effectively the same place where oxaloacetate binds during the backward reaction.
Fast forward a half century to this paper from 2013, and we learn much more:
Oxalate binds to the active site of the enzyme 15 times more strongly than pyruvate does.
Oxalate’s stereochemistry — which refers to the orientation of its molecular orbitals, essentially the geometric conformation of its electric charge — looks just like that of enolpyruvate, which is an intermediate in both the carboxylation of pyruvate and the decarboxylation of oxaloacetate.
Oxalate tucks one of its carboxyl groups right up right against threonine 882, the specific amino acid that donates a proton to enolpyruvate during the decarboxylation of oxaloacetate.
Pair this with what we knew from the studies I reviewed above:
Pyruvate carboxylase can run backwards.
Pyruvate carboxylase can metabolize other substrates, such as alpha-ketobutyrate.
Everything about this is screaming that pyruvate carboxylase can decarboxylate oxalate to form formate.
Yet I have not found one paper that so much as looked for this.
Suggestions From Other Enzymes
The bacterium Klebsiella aerogenes possesses a biotin-dependent oxaloacetate decarboxylase. The enzyme binds oxalate 30 times more strongly than oxaloacetate. The authors suggested, “Decarboxylation of oxaloacetate probably goes through an enolate transition state which would be perfectly resembled by oxalate and would thus explain its strong inhibitory power.”
It would be perfectly resembled by oxalate, but oxalate would not be decarboxylated? They did not address that issue explicitly, but I would think perfect resemblance of the transition state would lead to similarly perfect resemblance of the actual reaction.
More recently, a 2018 paper attempted to explain the “bi-functionality” of a “human oxaloacetate decarboxylase” known as furmaryl acetoacetate hydrolase. This enzyme will cleave fumarylpyruvate into fumarate and pyruvate and cleave acetylpyruvate into acetate and pyruvate, yet will also decarboxylate oxaloacetate to form pyruvate.
These authors did not investigate whether oxalate could be decarboxylated to formate because they were looking at the crystal structure of bound substrates and inhibitors.
They noted in the crystal structure that oxalate forms “a potential catalytic triad,” which means that it orients itself in the enzyme in a way that might lead to its catalysis.
That is, its decarboxylation to formate.
Thus, there is a general trend that oxaloacetate-metabolizing enzymes bind oxalate even more strongly and in a way that suggests it could be decarboxylated to formate.
Would Biotin Be Important?
Although biotin is a cofactor for pyruvate carboxylase, it is not automatically obvious that biotin would be needed for its decarboxylation of oxalate.
Biotin is an anabolic vitamin, and its role is to use the energy of magnesium-ATP to add carbon dioxide to molecules.
The way this happens in pyruvate carboxylase is as follows:
Biotin is carboxylated using Mg-ATP, to form carboxybiotin.
Carboxybiotin moves to a second location within the enzyme.
Biotin liberates the CO2 to form biotin enolate.
Biotin enolate takes a proton from a nearby threonine, which then takes a proton from pyruvate, forming enolpyruvate.
Enolpyruvate is unstable and it attacks the CO2 that biotin had liberated.
The joining of the CO2 to enolpyruvate forms oxaloacetate.
When the enzyme runs in reverse, oxaloacetate takes a proton from the threonine, rather than pyruvate giving it up. Energy is released rather than consumed. The energy is used to make magnesium come together with ADP and phosphate to form Mg-ATP.
Biotin is needed to help invest energy into carboxylation.
Would it have a role in decarboxylation?
Maybe! Even if it isn’t doing it’s typical job, it is still part of the machinery. Even if you are driving downhill, your engine is still on, for example. If you had no car engine you wouldn’t be coasting downhill for very long.
A 1974 paper found that avidin, which binds to biotin, does not completely inhibit the decarboxylation of oxaloacetate in the way it completely inhibits the carboxylation of pyruvate, but it does double the amount of oxaloacetate needed to get the same rate of decarboxylation.
In other words, an absence of biotin would not destroy your ability to decarboxylate oxalate, but it would double the amount of oxalate needed in circulation to get the same amount of detoxification.
So, if I am right about this, biotin would promote oxalate clearance.
If it promotes oxalate clearance, it could perhaps lead to temporary symptoms of what Owens calls “dumping.”
The Bottom Line
It has never been demonstrated that pyruvate carboxylase can decarboxylate oxalate. However, numerous lines of circumstantial evidence suggest that it is likely to participate in the first step of oxalate detoxification, its conversion to formate. This would allow formate to enter the methylation cycle or be converted to CO2. Together, this would allow oxalate to be broken down into two molecules of CO2 and exhaled.
If this is correct, biotin could help promote oxalate clearance. This may suggest a need to go slow in some cases where rapid clearance would lead to temporary bouts of uncomfortable symptoms.
This is over and above what Owens and Norton note, which is more clearly documented: that oxalate inhibits biotin-dependent enzymes at physiologically relevant concentrations.
The fact that Owens and Norton list milligram doses of biotin as helpful for clearance symptoms suggests to me that it is usually more relevant that oxalate is hurting the normal textbook functions of the biotin-dependent enzymes than it is that biotin provokes more rapid clearance, and as a result it is more often helpful to have higher doses of biotin during the period of clearance than it is to experience adverse effects from them.
However, my hypothesis leads to two important insights:
Biotin deficiency may directly lead to oxalate intolerance.
In the occasions where biotin supplementation leads to skin rashes, it may not always be about balance with other nutrients, but it could, perhaps, be directly supporting more rapid oxalate clearance.
More On Biotin
High Protein? You Need More Biotin
Most of us probably aren't getting enough biotin, and this is especially true if we are eating a lot of protein. These waters are murky, but here's my best estimate of how much we need.
Biotin Causes a Multitude of False Lab Tests | Here is What to Do
High-dose biotin supplements can cause a multitude of false lab tests, masking recent heart attacks, pregnancies, or allergies, giving false signals about tumors, and far more. Here is what to do.
When High-Dose Biotin Is Truly Needed
High-dose biotin is used for treatment of diabetes, disorders of the hair, skin, and nails, multiple sclerosis, and more. Are the high doses necessary?
My simple food-based recommendations for getting enough biotin are found in my Cliff Notes (free for Masterpass members here).
What Is Your Experience?
Do you have anything to share in your health journey on the interactions between biotin and oxalate? If so, let me know in the comments!
Stay Immune Through the Winter
My new 7-page quick guide on how to not get sick this winter, Staying Immune Through the Winter, is free for everyone. All you need is a free or paid subscription to my Substack.
Input your email below and immediately get my 7-page guide to not getting sick this winter!
Input your email below and immediately get my 7-page guide to not getting sick this winter!
If the box above says “subscribed,” this means you are a Masterpass member and can download the guide here.
If the box says “upgrade to paid,” then you are a free subscriber and your guide is currently sitting in your inbox. Search your mail, spam, and trash for “staying immune through the winter” and make sure anything from Substack is going to primary.
If it allows you to enter your email address, do so, hit “subscribe,” and then find the email from “Chris Masterjohn, PhD” with the subject “Welcome to My Newsletter!” If you can’t find this, try searching for either of those terms and for “Substack.” At the top of the welcome email you will find your downloadable guide to not getting sick.
Join the Next Live Q&A
Have a question for me? Ask it at the next Q&A! Learn more here.
Join the Masterpass
Masterpass members get access to premium content (preview the premium posts here), all my ebook guides for free (see the collection of ebook guides here), monthly live Q&A sessions (see when the next session is here), all my courses for free (see the collection here), and exclusive access to massive discounts (see the specific discounts available by clicking here). Upgrade your subscription to include Masterpass membership with this button:
Learn more about the Masterpass here.
Please Show this Post Some Love
Please like and share this post on the Substack web site. This will help spread it far and wide, as subscriber likes drive the Substack algorithm. Likes from paid subscribers count the most, but likes from free subscribers come in the highest numbers and are the foundation for spreading the content.
Take a Look at the Store
At no extra cost to you, please consider buying products from one of my popular affiliates using these links: Paleovalley, Magic Spoon breakfast cereal, LMNT, Seeking Health, Ancestral Supplements, MASA chips. Find more affiliates here.
For $2.99, you can purchase The Vitamins and Minerals 101 Cliff Notes, a bullet point summary of all the most important things I’ve learned in over 15 years of studying nutrition science.
For $10, you can purchase The Food and Supplement Guide for the Coronavirus, my protocol for prevention and for what to do if you get sick.
For $10, you can purchase Healing From COVID Vaccine Side Effects for yourself or a loved one if dealing with this issue. It also contains an extensive well-referenced scientific review, so you can also use this just to learn more about my research into the COVID vaccines.
For $29.99, you can purchase a copy of my ebook, Testing Nutritional Status: The Ultimate Cheat Sheet, my complete system for managing your nutritional status using dietary analysis, a survey of just under 200 signs and symptoms, and a comprehensive guide to proper interpretation of labwork.





Hi, Chris,
When I first began to obtain the background I needed to understand the oxalate issues I found in autism 18 years ago (2005), I attended professional oxalate conferences. I was very surprised to discover they were only attended by kidney doctors and kidney researchers. To them, finding oxalate elevated in autism was an anomaly. In fact, finding oxalate elevated apart from kidney stone disease or genetic hyperoxaluria was in their minds, impossible.
Why did I start studying it? Beginning about thirty years ago, my academic work was all about sulfate and discovering its role in neurodevelopment. Years later, sulfate was found to exchange for oxalate on a special set of transporters called the SLC26A family of transporters.
I had already thoroughly explored the role of sulfate in autism, but this new science meant that now I needed to study oxalate to find out how oxalate could disrupt sulfate and other things important in autism. To accomplish that, I studied the oxalate literature for four months with a special focus on the genetic hyperoxalurias where it was known that oxalate traveled to the whole body.
I attended 7 professional oxalate conferences. To my surprise, the scientists there seemed to have formed an unspoken pact to ignore issues that were not centered around kidney stone disease. They were uninterested in how oxalate affected the general biochemistry wherever it went. I had already spent a decade recognizing complex issues in autism, so I knew that oxalate, by affecting sulfate, could alter neurodevelopment as well as hormone and neurotransmitter regulation, and it was a clear mitochondrial toxin. This new information required a reinterpretation of autism findings.
Before that, I had been studying sulfate's role, but now, oxalate could be a major disruptor. Formate was also a substrate of those transporters. Anyone can find literature about how formate and oxalate have been influencing each other, but I found nothing practically useful.
Chris, this is why I am particularly delighted to find your interest in this field and I like the way you are looking at it in the way it should have been studied a long time ago.
The oxalate field for so many years had blinders on...something that Sally Norton and I had both found and explored independently. Sally has been more into thinking about issues with diet, but I became more concerned about reaching academia and helping them study the oxalate the body makes under stress.
It was impossible to do a study in the US on oxalate in autism because the labs in the US that were skilled in measuring oxalate told me they would not test anyone unless they had kidney disease. That is why I recruited a highly qualified group of scientists to look at oxalate levels in autism, and we got astonishing results. That paper is ranked now in the top 4% of scientific studies and it has had 42 citations. That attention is really great, but it is still not motivating other scientists to look past the kidneys and find other types of diseases related to oxalate.
Chris, the commendable sort of thinking that you are doing is exactly what is needed to move oxalate research forward and into new territory. I eagerly invite you to further explore this fruitful area that has been in sore need of your skill set. I hope that you can generate experimental evidence.
In 2005, with a bunch of grateful autism parents, we formed the TryingLowOxalates network and it has has already helped more than 70,000 people discover the relevance of oxalate to their own health. This is the way we picked up an enormous amount of personal experience about reducing oxalate, but we have not seen nearly the amount of change we want to see in academia.
We're trying to change that. Our group performed the most successful fundraising of anybody this past year for the Oxalosis and Hyperoxaluria Foundation for Giving Tuesday, and that is because our vision is to support the sort of thinking you are doing to move the study of oxalate into a new era, where multidisciplinary and cross-disciplinary work rules the roost instead of being barred from the roost.
Kudos on your deep thinking! I would be delighted to see people like you get funded so that we can together solve the many human diseases related to oxalate.
Best wishes,
Susan Owens
Head of the Autism Oxalate Project at the Autism Research Institute
Founder of the Trying Low Oxalates network since 2005
You are so amazing! I am so glad I fumbled my way into your orbit...