We Really Can Make Glucose From Fatty Acids After All! O Textbook, How Thy Biochemistry Hast Deceived Me!
Biochemistry textbooks generally tell us that we can't turn fatty acids into glucose. For example, on page 634 of the 2006 and 2008 editions of Biochemistry by Berg, Tymoczko, and Stryer, we find the following:
Animals Cannot Convert Fatty Acids to Glucose
It is important to note that animals are unable to effect the net synthesis of glucose from fatty acids. Specficially, acetyl CoA cannot be converted into pyruvate or oxaloacetate in animals.
In fact this is so important that it should be written in italics and have its own bold heading! But it's not quite right. Making glucose from fatty acids is low-paying work. It's not the type of alchemy that would allow us to build imperial palaces out of sugar cubes or offer hourly sweet sacrifices upon the altar of the glorious god of glucose (God forbid!). But it can be done, and it'll help pay the bills when times are tight.
All Aboard the Acetyl CoA!
When we're running primarily on fatty acids, our livers break the bulk of these fatty acids down into two-carbon units called acetate. When acetate hangs out all by its lonesome like it does in a bottle of vinegar, it's called acetic acid and it gives vinegar its characteristic smell. Our livers aren't bottles of vinegar, however, and they do things a bit differently. They have a little shuttle called coenzyme A, or “CoA” for short, that carries acetate wherever it needs to go. When the acetate passenger is loaded onto the CoA shuttle, we refer to the whole shebang as acetyl CoA.
As acetyl CoA moves its caboose along the biochemical railway, it eventually reaches a crossroads where it has to decide whether to enter the Land of Ketogenesis or traverse the TCA cycle. The Land of Ketogenesis is a quite magical place to which we'll return in a few moments, but navigating the TCA cycle can be a nightmare. Traveling down this route is particularly dreadful for three reasons. First, every time the Biochemical Traffic Committee holds session it has the the cycle renamed and has the signage repainted. As a consequence, everyone is always calling it something different. Some call it the tricarboxylic acid cycle. Others call it the citric acid cycle and yet others call it the Krebs cycle. Second, the TCA cycle is a treacherous roundabout. It puts the typical town traffic circle to shame with its eight exits, all replete with incoming and outgoing traffic. Third, even our cheerful little CoA That Could has to feel a tinge of guilt gliding along a railway that's been used to mercilessly torture generations of memorization-impaired biochemistry students in universities everywhere.
If acetyl CoA navigates the TCA cycle flawlessly, completing a full turn of the circle without either getting into a traffic accident or wandering off along one of its myriad exits, it arrives at that sacred space wherein our cells make new glucose. Presuming a bit of poetic license, let's call this space the Candy Factory.
Balancing the Carbon Accounts in the TCA Cycle
But now we come to the problem that biochemistry textbooks grapple with so simplistically, and as we'll see, so wrongly: it is mathematically impossible for acetyl CoA to yield a net synthesis of glucose when it arrives at the Candy Factory by this route. In other words, it is impossible by this means for acetate to contribute to the production of more glucose than is used up just to keep the TCA cycle running. We can see this illustrated in the following flow chart, taken from a 1957 review that discussed this matter in detail (1):
This diagram is greatly simplified so that we can see just the essential points. On the left, we see the point where glucose enters or exits the cycle. When more glucose exits the cycle than enters it — that is, when more glucose is produced than consumed — we are in a state of gluconeogenesis and our Candy Factory is fully operational. On the top, we see the point where acetyl CoA enters the cycle. Acetate is a two-carbon molecule, so it naturally brings only two carbons to the table. On the bottom, we see that both of these carbons leave the cycle as carbon dioxide before the CoA train even reaches the Candy Factory station. Two minus two is zero, so there are no carbons left for making glucose.
The only way to make sure acetate carbons get stuffed into any of the delectable delights produced in the Candy Factory is for other molecules to enter the TCA cycle at any of the many entry points not shown in the above diagram and thereby provide those two carbons that need to leave the cycle as carbon dioxide during each turn. Indeed, careful experiments using radioactive tracers had already definitively shown that carbons could flow from fatty acids to glucose in this manner by the time that review had been published in 1957.
But that's not a net synthesis of glucose. For every two carbons that fatty acids could provide for glucose synthesis in that scenario, two would have to be taken either from glucose itself, or from some other molecule that could just as easily have served as a precursor to glucose, just to keep the TCA cycle going. Once again, two minus two is zero, and fatty acids cannot contribute to the net synthesis of new glucose in this manner.
Magical Things Happen In The Land of Ketogenesis
By the time the 1980s rolled around, however, it had become clear that fatty acid metabolism is more complex than this and that there are indeed ways that fatty acids can contribute to the net synthesis of new glucose.
When large quantities of fatty acids flood the liver during fasting, caloric restriction, diabetes, or the consumption of a low-carbohydrate, high-fat, ketogenic diet, our livers produce so much acetate that the TCA cycle suffers heavy traffic. Any acetyl CoA with the foresight to listen to the evening traffic report would quickly decide to head straight for the Land of Ketogenesis, where the railways are open and the paths are free. This is where our livers turn acetate into ketones, sending the ketones out into the bloodstream so our other tissues can use them for energy.
One of the ketones we make is acetone. Acetone makes an excellent paint thinner, and is responsible for the “ketone breath” that some people get on low-carbohydrate diets. It also happens to be a great raw material for making glucose.
In 1979, a group of researchers from Philadelphia studied acetone metabolism in fasting humans (2). These authors estimated that during a three-day fast acetone may constitute over a third of the ketones we produce, and that 50-70 percent of it undergoes further metabolism. They used radioactive tracers to show that acetone could be converted to glucose in these subjects, and estimated that if in fact acetone contributes to the net synthesis of new glucose, it could account for just over ten percent of such glucose newly made.
In the mid-1980s, researchers showed that rats can convert acetone to glucose through two different intermediates, methylglyoxal and 1,2-propanediol (3). These pathways were summarized graphically in a later review (4):
The pathways shown above represent several alternative methods of converting acetone to pyruvate, which can then be converted to glucose.* Since acetone is formed from acetyl CoA, this directly contradicts the claims of even the most recent biochemistry textbooks, which plainly state as a matter of fact that “acetyl CoA cannot be converted into pyruvate or oxaloacetate in animals.” We would expect each of these pathways leading to pyruvate to result in the net synthesis of new glucose.
In 1986, researchers administered radioactively labeled acetone to rats and examined the radioactive carbon “fingerprint” on the glucose molecules formed from it to provide additional evidence that acetone followed pathways leading to pyruvate that would indeed lead to a net synthesis of new glucose (5). That same year, the Philadelphia group used a similar approach to show that acetone was following similar pathways in humans with diabetic ketoacidosis (6). They estimated that in such patients at least ten percent of newly synthesized glucose may come from acetone.
Blood levels of acetone also rise appreciably in adults on the Atkins diet (7) and in epileptic children following a ketogenic diet (8), suggesting that it may be a normal state of affairs for humans to convert fatty acids to glucose when consuming a diet low in carbohydrate and high in fat.
For comparison, I compiled the blood levels of acetone reached in humans under various conditions in the following table:$
In July of 2011, a German research group revisited the question of converting fatty acids to carbohydrate by publishing a computational analysis of the most up-to-date information about human biochemistry available (9). These authors identified 22 pathways by which acetone could be converted to pyruvate that they considered likely to be important, and concluded that these pathways would be less cost-efficient than making glucose from amino acids or glycerol, but are nevertheless biochemically feasible and likely serve as supplementary modes of glucose production.
Lo and behold, we have three decades of evidence suggesting that the Land of Ketogenesis is graced with its own Candy Factory. Sure, the desserts conjured therein may be sold at higher prices than those made in the Candy Factory just off exit eight of the TCA cycle, but when the traffic is heavy there, what else are we to do? Such is the law of supply and demand.
Insulin Regulates Gluconeogenesis From Fatty Acids
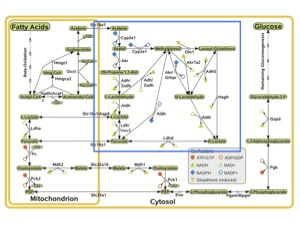
If we really do make glucose from fatty acids when times are tight as all of this evidence so strongly suggests, there should be a way for our bodies to regulate this process so that it only kicks in when we are in need of glucose. Indeed, such a mechanism exists. Let's take a look at a figure from the recent computational analysis (9) and focus in on the part I outlined in blue:
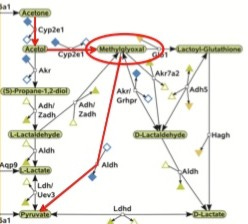
This part of the picture represents a complicated network of reactions that provide a multitude of ways to achieve the critical event needed to convert fatty acids to glucose: the conversion of acetone to pyruvate (you know, that conversion that the biochemistry textbooks categorically state can never happen). Pyruvate is half of a glucose molecule, so once acetone has made it that far, the rest is downhill. Let's zero in on this part of the picture and pay special attention to the part I circled in red:
We can see that despite the many different paths down which acetone may travel to ultimately wind up at pyruvate, they all start with the conversion of acetone to acetol, a conversion facilitated by an enzyme called cytochrome P450 2E1, or CYP2E1 for short. Insulin suppresses the production of this enzyme, while acetone prevents its degradation (10). Thus, when insulin levels fall and ketone levels rise, as occurs when our carbohydrate intake is low, our cells increase their supply of CYP2E1 and thereby activate the conversion of fatty acids to glucose. We've found our way to the expensive Candy Factory in the magical Land of Ketogenesis.
The authors of the computational analysis (9) calculated that the most cost-efficient way of converting fatty acids to glucose is by converting acetol to methylglyoxal, facilitated again by CYP2E1, and then converting methylglyoxal directly to pyruvate, facilitated by an enzyme called aldehyde dehydrogenase. I've outlined this pathway in red here:
As Peter Dobromylskyj over at Hyperlipid has pointed out before, methylglyoxal inhibits the breakdown of glucose. In a future post, I will cover methylgyloxal's inhibition of glucose consumption in greater detail, but for now it is worth noting that when this pathway is activated, we not only convert fatty acids to glucose, but methylglyoxal concentrations rise and inhibit the breakdown of glucose. Thus, when glucose runs low and we begin subsisting primarily on fatty acids for fuel, we have a coordinated effort to both spare glucose and to make more of it. When the glucose recession hits, our cells do what any other budget-conscious cells would do and spend less.
O Textbook, Why Hast Thou Deceived Me?
In the 1980s, at least two reviews were published outlining the evidence for the conversion of fatty acids to glucose (4, 11). One of them emphasized that biochemistry students were taught that such pathways do not exist (11):
Students are often asked to describe a pathway by which a long-chain fatty acid is converted into glucose in mammalian liver. This type of question is normally a trick one which, for most biochemists, would have a simple reply: such a pathway does not exist. Nevertheless, recent studies point towards a role for acetone in the conversion of fat to carbohydrate.
It further emphasized that most textbooks wrongly classify acetone as a useless chemical that can't be metabolized at all (11):
Most biochemistry textbooks state that acetone is a non-metabolizable byproduct of lipid metabolism, which accumulates when there are insufficient glycolytic intermediates to effect the complete oxidation of the acetyl CoA generated in the degradation of fatty acids. However, studies with 14C-labeled acetone in lactating cows, rats, guinea pigs, and humans have shown that acetone is not just excreted, but that it can be metabolized further.
The other review (4) noted that the first evidence for the conversion of acetone to glucose had been generated as far back as 1951.
Another quarter century has gone by, and the textbooks haven't changed their tune one toot. At the beginning of this post, I quoted Biochemistry by Berg, Tymoczko, and Stryer, which plainly states in a boldfaced section header that “Animals Cannot Convert Fatty Acids to Glucose.” Of acetone, all this book tells us is that its odor can be detected in the breath. Another biochemistry textbook I keep on hand, the 2005 edition of Lippincott's Illustrated Reviews: Biochemistry, tells us that acetone is a “non-metabolizable side product” of ketone production.
What is most striking is that these textbooks do not even alert us to any controversy about this topic, let alone to the strong evidence supporting the opposing view. This emphasizes the need to use what we learn from textbooks and academic classes as a starting point for further exploration of the primary literature. If we don't have time for that, as is usually the case, we need to seek out the best arguments from opposing viewpoints and consider them with an open mind. If we do not have even the time for that, I think it would be wise not to cling too tightly to any of our cherished beliefs. If this applies to something as mundane as whether acetone can be converted to glucose, it must hold true all the more for the myriad topics that have emotion, politics, money, or ideology lurking within them.
None of this is to say that we should blame the authors of these textbooks. Rather, we should admire them for undertaking such a gargantuan task, a task that no human or small group of humans could execute flawlessly. What is important is the recognition that any such work, no matter how authoritative, is a human work and thus necessarily subject to error.
The Good News
On the bright side, this finding is a testament to the great versatility of life. Biochemistry is enormously complex, and while activating any particular set of pathways might not necessarily be optimal, the plethora of possibilities contributes to the resiliency we possess as living beings.
And three cheers for the Little CoA That Could!
Join the Next Live Q&A
Have a question for me? Ask it at the next Q&A! Learn more here.
Subscribe
Subscribe or upgrade your subscription here.
Join the Masterpass
Masterpass members get access to premium content (preview the premium posts here), all my ebook guides for free (see the collection of ebook guides here), monthly live Q&A sessions (see when the next session is here), all my courses for free (see the collection here), and exclusive access to massive discounts (see the specific discounts available by clicking here). Upgrade your subscription to include Masterpass membership with this link.
Learn more about the Masterpass here.
Take a Look at the Store
At no extra cost to you, please consider buying products from one of my popular affiliates using these links: Paleovalley, Magic Spoon breakfast cereal, LMNT, Seeking Health, Ancestral Supplements. Find more affiliates here.
For $2.99, you can purchase The Vitamins and Minerals 101 Cliff Notes, a bullet point summary of all the most important things I’ve learned in over 15 years of studying nutrition science.
For $10, you can purchase The Food and Supplement Guide for the Coronavirus, my protocol for prevention and for what to do if you get sick.
For $29.99, you can purchase a copy of my ebook, Testing Nutritional Status: The Ultimate Cheat Sheet, my complete system for managing your nutritional status using dietary analysis, a survey of just under 200 signs and symptoms, and a comprehensive guide to proper interpretation of labwork.
Notes
* This graph as originally published included a direct conversion of acetone to acetate and formate, and conversion of this acetate to glucose. Acetate would be converted to glucose through the TCA cycle and would thus not yield net synthesis of new glucose. I erased this pathway because we currently do not have evidence for acetone being converted to acetate without first being converted to pyruvate (Christoph Kaleta, senior author of reference 9, personal communication). In any case, the authors discussed evidence that this pathway is not active unless supraphysiological doses of acetone were given. In the latter case, they may have been observing conversion of pyruvate to acetate when the capacity for gluconeogenesis from pyruvate was saturated.
$ It is important to realize that 1) these values are taken from different studies over the span of 27 years using different experimental techniques, 2) none of these subjects were randomly allocated to specific treatments, and 3) plasma acetone will be influenced as much by acetone metabolism and excretion as by acetone production. This table is therefore provided for purposes of rough comparison only, and no conclusions should be drawn about relative acetone production under these different conditions. Moreover, inter-individual variation is not represented in this table. See references for standard deviations, standard errors, or ranges given therein.
References
1. Weinman EO, Strisower EH, Chaikoff IL. Conversion of fatty acids to carbohydrate; application of isotopes to this problem and role of the Krebs cycle as a synthetic pathway. Physiol Rev. 1957;37(2):252-72.
2. Reichard GA Jr, Haff AC, Skutches CL, Paul P, Holroyde CP, Owen OE. Plasma acetone metabolism in the fasting human. J Clin Invest. 1979;63(4):619-26.
3. Casazza JP, Felver ME, Veech RL. The metabolism of acetone in the rat. J Biol Chem. 1984;259(1):231-6.
4. Landau BR, Brunengraber H. The role of acetone in the conversion of fat to carbohydrate. Trends in Biochemical Sciences. 1987;12:113-4.
5. Kosugi K, Chandramouli V, Kumaran K, Schumann WC, Landau BR. Determinants in the pathways followed by the carbons of acetone in their conversion to glucose. J Biol Chem. 1986;261(28):13179-81.
6. Reichard GA, Jr, Skutches CL, Hoeldtke RD, Owen OE. Acetone metabolism in humans during diabetic ketoacidosis. Diabetes. 1986;35(6):668-74.
7. Beisswenger BG, Delucia EM, Lapoint N, Sanford RJ, Beisswenger PJ. Ketosis leads to increased methylglyoxal production on the Atkins diet. Ann NY Acad Sci. 2005;1043:201-10.
8. Musa-Veloso K, Likhodii SS, Rarama E, Benoit S, Liu YM, Chartrand D, Curtis R, Carmant L, Lortie A, Comeau FJ, Cunnane SC. Breath acetone predicts plasma ketone bodies in children with epilepsy on a ketogenic diet. Nutrition. 2006;22(1):1-8.
9. Kaleta C, de Figueiredo LF, Werner S, Guthke R, Ristow M, Schuster S. In silico evidence for gluconeogenesis from fatty acids in humans. PLoS Comput Biol. 2011;7(7):e1002116. Epub 2011 Jul 21.
10. Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos. 2007;35(1):1-8.
11. Argiles JM. Has acetone a role in the conversion of fat to carbohydrate in mammals? Trends in Biochemical Sciences. 1986;11(2):61-63.









2 Acetyl-CoA -> BHB -> Acetoacetate -> acetone + CO2
If acetone can be converted to acetol, then it can also be converted by the same method to another useful substrate, dihydroxyacetone, which would become phosphorylated.