Erythritol and Blood Clotting
An analysis of the recent study claiming erythritol contributes to cardiovascular disease.
Masterpass members have access to a monthly Q&A that includes a question submission contest, and one of runners up for tonight’s AMA asked me to take a look at the new study in Nature Medicine tying erythritol levels to cardiovascular risk.
This is brought to us by the Hazen group at Cleveland Clinic, the folks who brought to us the research tying TMAO to cardiovascular disease.
The study found the following:
In two separate cohorts with a high prevalence of diabetes and adiposity, plasma erythritol was higher among those with cardiovascular disease and major adverse cardiovascular events.
Erythritol increased the propensity of the blood to clot in human blood in vitro and in a live mouse model of injury to the carotid artery, which feeds the brain.
Consumption of a drink with 30 grams of erythritol by human volunteers elevated erythritol beyond the levels needed to cause clotting in vitro and in live mice for more than a full day.
This article explains the two major metabolic pathways involved, the polyol pathway and the pentose phosphate pathway, explains why erythritol is likely to have encouraged platelet activation in vitro and when injected into live animals, and takes a look at whether the use of erythritol as a sweetener is likely to contribute to cardiovascular disease. In particular, the potential of sugar alcohols to contribute to osmotic stress and the potential for erythritol to serve as a marker of thiamin deficiency are highlighted.
Masterpass members have access to the article now and can attend a live video explanation in tonight’s AMA. This article will then be made available to the public on Thursday, March 16.
There are two major biocehmical pathways needed to understand erythritol metabolism in humans. The first is the polyol pathway, and the second is the pentose phosphate pathway.
The Polyol Pathway
In organic chemistry, the suffix “-ol” refers to an alcohol, which is something that contains a hydroxyl group, made of one atom of oxygen and one atom of hydrogen (OH). A polyol is a compound with multiple OH groups.
In biochemistry and food science, polyols tend to refer more specifically to a subset known as "sugar alcohols.” These are distinct from “sugars” in having all of their oxygens participating in OH groups, whereas sugars have one of their oxygens participating in an aldehyde or ketone group, which are two types of carbonyl groups. Carbonyl groups consist of a an oxygen without any hydrogens, and the distinction between them is about the location on the overall sugar that they occupy. Specifically, aldehyde groups are on the end of a molecule and ketone groups are somewhere in the middle of a molecule, that is, anywhere except the ends.
For example, glucose has its carbonyl group (C=O) on the end of the molecule, on carbon 1:
Therefore, it is an aldehyde sugar. Sugars bear the suffix “-ose.” Since glucose is an aldehyde, it is also known as an aldose. It has six carbons, so it is also known as hexose. Combining these, it is also known as a aldohexose.
Galactose is also an aldehyde, aldose, and aldohexose, and is known as a “4-epimer” of glucose because the OH on the fourth carbon is oriented oppositely, shown in these figures as pointing to the left rather than the right:
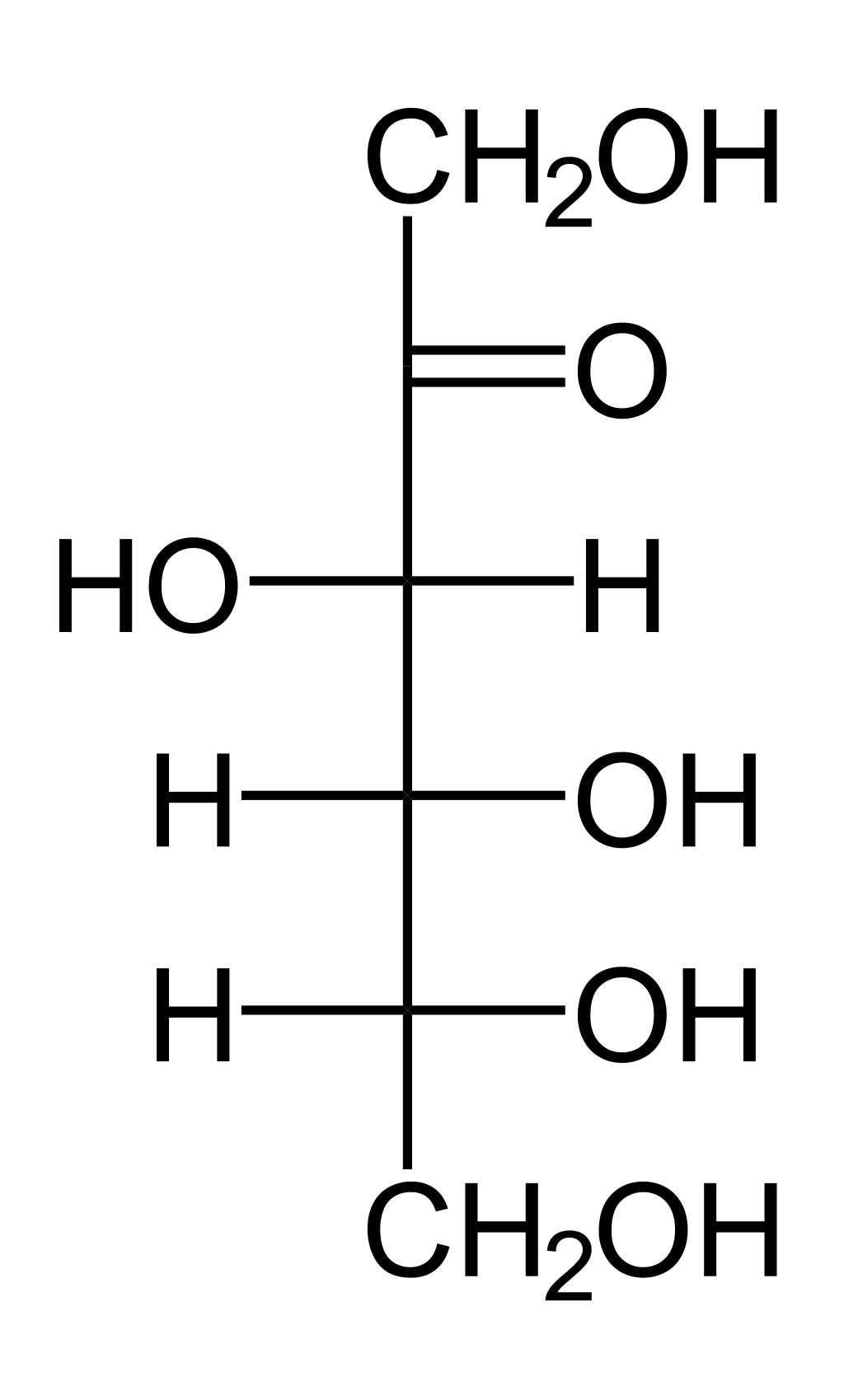
Fructose has its carbonyl (C=O) on the second carbon, which is not on either end:
Therefore, fructose is a ketone, a ketose, and a ketohexose.
Erythrose is a four-carbon aldehyde sugar, making it an aldehyde, a tetrose, and an aldotetrose:
The polyol pathway converts sugars to their corresponding sugar alcohols by adding hydrogen atoms and electrons to the carbonyl groups, reducing them to hydroxyl groups, using material and energy derived from glucose in the pentose phosphate pathway, carried by a derivative of vitamin B3 or niacin in the form of NADPH. This reaction is catalyzed by the enzyme aldose reductase or a similar enzyme from a large family of aldo-keto reductases.
In this pathway, the following conversions, and many analogous conversions from other sugars, take place:
Glucose to sorbitol.
Galactose to galactitol.
Erythrose to erythritol.
Since the polyol pathway uses NADPH, it has the potential to deplete NADPH from other purposes, such as the recycling of vitamin K, folate, and glutathione. As explained in lessons 6, 7, 10, and 11 of my Antioxidant System course, the recycling of glutathione by NADPH represents the fueling of the entire antioxidant system by the system of energy metabolism. By taxing the NADPH needed for glutathione recycling, therefore, activation of the polyol pathway has the potential to contribute to oxidative stress.
Sugar alcohols can also contribute to osmotic stress, which is the drawing of water to one or another side of a membrane, contributing to cellular dehydration or swelling. This is because they do not cross membranes through transporters as readily as sugars do. So, wherever they are, they draw water, with less opportunity for the cell to defend against this by equilibrating their concentrations across either side of the membrane.
The polyol pathway is a spillover pathway for sugars that exceed the capacity of cells to metabolize them through the normal routes.
For example, if we looks at the correlation of the concentrations of glucose in plasma and sorbitol in cerebrospinal fluid (CSF), in controls (blue circles), those with elevated CSF glucose (green squares) and type 2 diabetics (purple diamonds), we see that sorbitol seems to start increasing above normal around 8 millimoles per liter (mmol/L) glucose, which is 144 milligrams per deciliter (mg/dL):
Using insulin to bring plasma glucose down below 140 mg/dL reduces plasma sorbitol down to the level of healthy controls. This is almost certainly not an effect of bringing glucose from plasma into cells — where it could be turned into sorbitol! — but rather the stimulation of the burning of glucose for energy using glycolysis, the citric acid cycle, and the respiratory chain, as explained in Lesson 24 of my Energy Metabolism course.
We must remember here that glucose transporters moved to the plasma membrane of muscle and adipose cells by insulin do not lead to net transport of glucose into cells, because glucose transporters are completely reversible and bidirectional. Insulin bringing GLUT4 to cell membranes simply makes glucose transport occur faster. It does not change the direction of the transport. What does lead to the net transport of glucose into cells is the trapping of glucose as glucose 6-phosphate and its downstream metabolism for energy, all of which make a gradient across the membrane where free glucose inside the cell is kept at very low concentrations compared to free glucose in the plasma. This and only this is what favors net transport of free glucose into cells through glucose transporters, and this is just as stimulated by insulin as the moving of GLUT4 transporters to the cell membrane. And it is this that also prevents intracellular glucose from spilling into the polyol pathway.
Sorbitol levels are inversely correlated with glutathione levels in red blood cells, supporting the framework whereby excess glucose spills into the polyol pathway, sapping the NADPH needed for glutathione recycling, thereby contributing to oxidative stress.
When someone first starts drinking milk, they have not yet increased the enzymes they need to metabolize galactose, and many people have a short-lived rise in galactitol.
In this study, individuals were separated into two groups based on how high their plasma galactose rose after ingesting 0.5 grams per kilogram per day of a galactose supplement. Those who were deemed “galactose intolerant” had peak plasma galactose over 40 mg/dL, whereas those deemed “galactose tolerant” had peaks under 30 mg/dL
As you can see in the following table the galactose intolerant individuals (group A), had a sharp rise in galactitol on day 1 that decreased almost 5-fold within the first week and completely disappeared by day 15.
This also corresponded to a dramatic decrease in the peak blood galactose across the first two weeks, which is likely mediated by increased expression of the enzymes needed for normal galactose metabolism, especially galactokinase, galactose 1-phosphate uridyl-transferase, and UDP-galactose 4’-epimerase. This ultimately allows galactose to enter glycolysis or the pentose phosphate pathway after its conversion to glucose 6-phosphate.
If the galactose cannot be phosphorylated, it also cannot be trapped inside cells, so its concentration in the blood rises. But, what does get in cells is also spilling over into the polyol pathway.
So, low expression of the machinery of intracellular galactose utilization leads to “galactose intolerance” and abnormal production of galactitol. Over two weeks of consuming galactose, this machinery dramatically ramps up, galactose intolerance is abolished, and galactose no longer activates the polyol pathway.
Now, something similar must be happening with the conversion of erythrose to erythritol, but to understand where the erythrose would be coming from, we need to understand the pentose phosphate pathway.
Production of Erythritol in the Pentose Phosphate Pathway
The pentose phosphate pathway is covered in detail in Lesson 27 of my Energy Metabolism course.
Its purpose is to load hydrogen ions, electrons, and energy onto NADP+ to be carried as NADPH for antioxidant defense and detoxification (glutathione recycling, cytochrome P450 enzymes), nutrient recycling (vitamin K and folate), and anabolic synthesis (fatty acids, cholesterol, neurotransmitters, nucleotides), as well as 5-carbon sugars needed to make structures involved in growth, reproduction and repair (DNA), protein synthesis (RNA), and energy metabolism (NADH, NADPH, FADH2, CoA, and ATP).
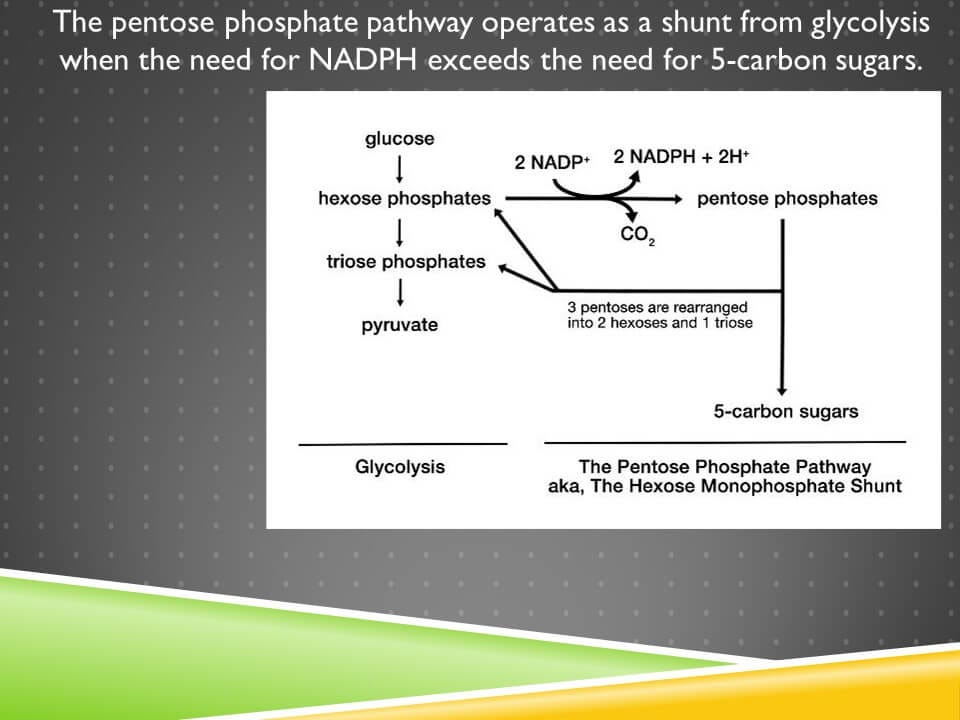
It acts as a shunt from glycolysis:
The conversion of NADP+ to NADPH occurs in the first phase, known as the oxidative phase. This leads to the production of 5-carbon sugars or pentoses. If pentoses are needed, they can be exported at this point. However, the shunt can be reconnected to glycolysis using the non-oxidative reactions where pentoses are rearranged into 6-carbon and 3-carbon sugars, hexoses and trioses.
This pathway is entirely regulated by the needs for NADPH and 5-carbon sugars. If the cell needs NADPH but does not need 5-carbon sugars, it will run the pathway in a loop, producing as much NADPH as it needs, and returning the sugars to glycolysis. If it needs 5-carbon sugars, it will use the pentoses without ever returning them to glycolysis.
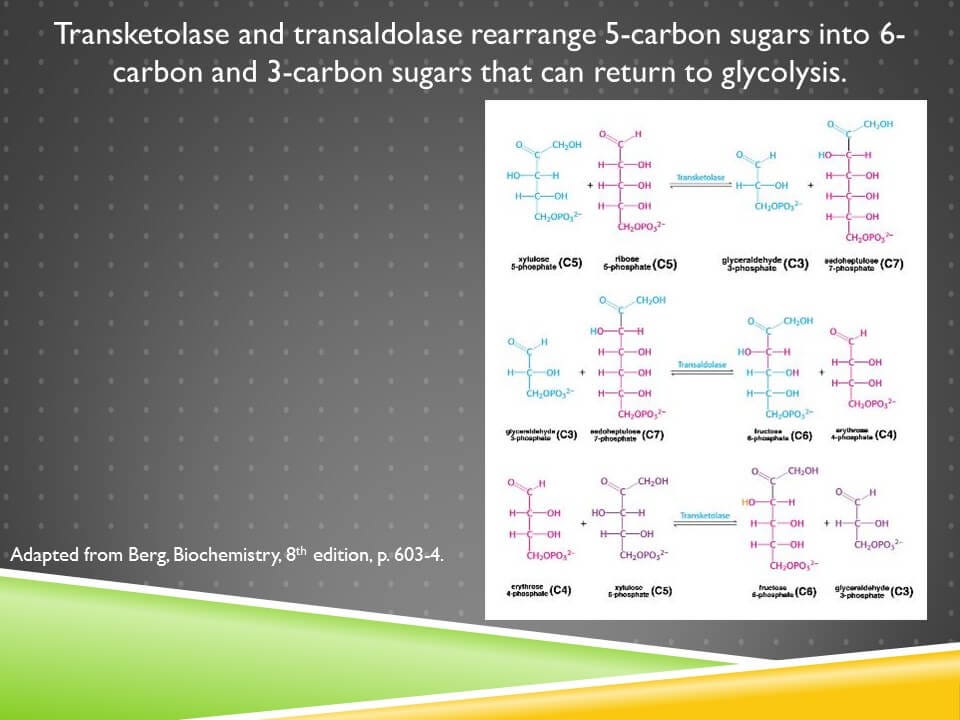
Erythrose 4-phosphate is produced during the rearrangement of the pentoses into 6-carbon and 3-carbon sugars:
There are two enzymes involved in this, transketolase and transaldolase, and the only nutritional cofactor needed at all here is thiamin, or vitamin B1, which is a cofactor for transketolase.
The entire point of this is not to make any erythrose 4-phosphate but rather to dispose of unneeded pentoses by returning them to glycolysis in the form of hexoses and trioses so that the oxidative phase of the pathway can be run in an endless loop to make more NADPH.
Thus, the main reason for erythrose 4-phosphate to elevate would be thiamin deficiency.
That is, there is enough transketolase activity to allow the production of erythrose 4-phosphate, but not enough to clear all of it to glycolysis.
Plasma erythritol is elevated in diabetics who develop retinopathy. Studies have also associated it with central adiposity and the onset of type 2 diabetes.
It is extremely unlikely that this results from a “push-forward” effect of high glucose, because there is no biochemical rationale for the pathway to be regulated in this way. Rather, deficient insulin signaling leads to the accumulation of free glucose that is not being metabolized for energy, while activation of the polyol pathway and de novo lipogenesis lead to the depletion of NADPH. This raises the pool of NADP+, which signals the need to run the pathway, and the NADP+ drives the pathway forward out of demand.
Erythritol and Thiamin Deficiency
Meanwhile, thiamin deficiency makes the pathway run sloppily and incompletely, with the second use of transketolase not keeping up with the first, and leading to the accumulation of erythrose 4-phosphate.
Biochemistry textbooks will not tell you that this can lead to erythritol production, nor will they tell you that erythritol can enter the pentose phosphate pathway. However, it is very likely that both of these are true.
A 2017 study found that those recently developing central adiposity had 15-times higher blood erythritol than those who had stable adiposity, and that those with HbA1c over 5.05% had 21-fold higher blood erythritol than those with lower HbA1c. They performed experiments showing that the pentose phosphate pathway was endogenously producing erythritol in these subjects.
This implies that erythrose 4-phosphate can be dephosphorylated to erythrose and reduced to erythritol.
A 2019 paper showed that erythrose can be reduced to erythritol using the enzymes alcohol dehydrogenase 1 and sorbitol dehydrogenase. As we would expect, this uses the energy, hydrogens, and electrons carried by NADPH.
My suspicion is that suboptimal thiamin status drives the accumulation of erythrose 4-phosphate, and then a phosphatase frees erythrose and the enzymes just described reduce it to erythritol. This adds fuel to the fire of NADPH depletion, further compromising the defense against oxidative stress, and upregulating the pentose phosphate pathway in a vicious cycle.
To my knowledge there are no studies demonstrating that plasma erythritol acts as a marker of thiamin deficiency or suboptimal thiamin status, but I believe the biochemistry predicts that it should.
Can Erythritol Sweeteners Be Utilized in the Pentose Phosphate Pathway?
The 2017 study showed that 5-10% of supplemental erythritol is metabolized to erythronate. This represents its oxidation to a carboxylic acid, analogous to the conversion of glucose 6-phosphate to 6-phosphogluconate in the first step of the pentose phosphate pathway.
This raises the possibility, but does not show clearly, that erythritol can be converted to erythrose and erythrose 4-phosphate. If it can, it would presumably enter the pentose phosphate pathway from there.
Given that no one expected erythritol to be produced by the pentose phosphate pathway until they looked for it, nor to be converted to erythronate until they looked for it, and given that one of the best biochemistry textbooks available (by Berg, Stryer, and colleagues) makes no mention of erythritol or erythronate, my strong suspicion is that erythritol can indeed be metabolized in this way.
When humans were fed 1 gram of erythritol per kilogram of bodyweight, 30% was found in the urine within 3 hours, and 78% was found during the first 24 hours. This implies that there are hours for the body to decide what to do with nearly all this erythritol, with various bottlenecks in metabolic pathways determining what happens to it. The authors concluded that most of the remainder of the erythritol “remained unabsorbed and was available for colonic fermentation and potential production of short-chain fatty acids,” but they didn’t measure fecal erythritol.
I don’t see how any conclusions about intestinal absorption of anything are ever valid if no fecal measurements are made (that is, in almost all such experiments).
In the 2017 study, “a large portion of consumed erythritol was assumed to be excreted in urine.”
So, the urine study assumes what doesn’t appear in the urine must be in the feces (why bother measuring when we can assume) and the study finding 5-10% conversion to erythronate assumes most of the rest is in the urine (why bother measuring when we can assume), meanwhile no one looked at whether metabolism into the pentose phosphate pathway is next in the long list of completely unexpected findings on erythritol metabolism.
Likely the ability to metabolize erythritol in this way is limited by factors controlling the activity of the pentose phosphate pathway, such as thiamin status.
The New Erythritol Study
The new study in Nature Medicine showed the following:
In two separate cohorts with a high prevalence of diabetes and adiposity, plasma erythritol was higher among those with cardiovascular disease and major adverse cardiovascular events.
Incubating platelet-rich plasma or isolated platelets with erythritol made the platelets more likely to aggregate in response to other stimuli, but glucose and 1,5-anhydroglucitol did not do this. 1,5-anhydroglucitol is a polyol that is used as an inverse marker of glycemic control.
Human whole blood was more likely to show platelets adhering together when subject to shear stress if erythritol was added to it.
In a mouse model of carotid injury that took place over the course of 8 minutes, the blood appeared to clot faster when the mice where injected with erythritol, but not when they were injected with saline or with 1,5-anhydroglucitol.
Consumption of a drink with 30 grams of erythritol by human volunteers elevated erythritol beyond the levels needed to cause clotting in vitro and in mice for more than a full day.
The authors did not measure erythritol intake, so there is no particular reason to believe that the observational findings reflect anything more than endogenous activation of the pentose phosphate pathway with an extremely sloppy non-oxidative phase, mediated by NADPH depletion and suboptimal thiamin status as described above.
They did adjust for age, sex, diabetes mellitus, low-density and high-density lipoprotein cholesterol levels, triglyceride levels and current smoking status, and, depending on the cohort, systolic blood pressure and BMI. However, this doesn’t adjust for poor control of diabetes or for the degree of sloppiness in the activation of the pentose phosphate pathway. As noted above, within diabetes, plasma erythritol predicts diabetic retinopathy. Thus, simply adjusting for diabetes does not control the relevant variables.
Why Would Erythritol Cause Platelet Activation?
The authors made no attempt to determine the mechanism by which erythritol caused platelet activation in any of their experiments.
Given that no mechanism specific to erythritol is known, it follows that we should use as a working model what is already known about platelet activation that could easily explain it in a rather generic way.
In 1996, researchers were trying to determine why some types of contrast media were causing platelet aggregation, so they mixed together various ingredients of solutions to control for omsolality and ionic strength. Osmolality is the concentration of dissolved solutes defined by the number of solute particles rather than mass, while ionic strength is the concentration of dissolved ions. Most of the effect was due to increasing osmolality, with a small effect of decreasing ionic strength:
The effect of osmolality is dramatic during the initial rise from normal plasma and cell culture conditions (260-320 mOsm/kg).
While the pro-clotting effects in the Hazen study were shown with only 45-270 micromoles per liter, which would represent considerably less than 1 mOsm/kg and thus seem rather insignificant, a key distinction must be made between erythritol and the other solutes: membranes are extremely slow at transporting erythritol.
The major osmolytes in human plasma are glucose and sodium. The clotting experiments in the Hazen paper were done with plasma, whole blood, or platelets cultured in Hank’s buffered salt solution, where the major osmolytes are glucose and various mineral salts. In the mice, the major osmolytes would again be the glucose and sodium of their own blood.
Glucose sodium, and other electrolytes, are transported across the membranes of cells and platelets according to the needs of those cells, and, in a live organism, the need of the organisms to maintain the characteristics of circulating blood.
Among these needs are controlling osmotic pressure. Therefore, a cell or platelet can rather easily defend itself against osmotic stress simply by controlling how much glucose or salt it takes in.
This is not true with erythritol. Why?
The permeability of a red blood cell to erythritol is about 1000 times slower than the permeability to glucose, and this is because erythritol has very low affinity for glucose transporters and for the enzymes that phosphorylate glucose to trap it inside the cell.
On the other hand, 1,5-anhydroglucitol is similar in size and structure to glucose, and has roughly half the affinity for the transport system as glucose does, although less than 3% of it gets burned for energy.
Platelets express glucose transporters, using mainly GLUT3, typically otherwise associated with neurons, which has a five-fold greater transport capacity than the more common glucose transporters GLUT1 and GLUT4. This means that both glucose and 1,5-anhydroglucitol will be rapidly transported into platelets while erythritol will not, and that erythritol would thus be much more likely to serve as an osmolyte outside the platelets, thereby contributing to their activation.
Erythritol would also be likely to decrease the ionic strength of the solution, since platelets or cells would presumably respond to osmotic stress by bringing charged ions into the cell and out of the blood or culture media.
In fact, this is very similar to how hyperglycemia can cause hyponatremia: in response to elevated osmolality, the kidneys will remove sodium from the blood to bring osmolality back to normal.
The chief problem with adding erythritol to platelets in vitro is that the experiment gives no chance for human physiology to step in and regulate osmolality. Adjusting 270 micromoles/liter of an impermeable solute could be done by removing 5 mg/dL of glucose from the blood, or less, if the concentration of other solutes were also adjusted.
When rats consume between 2% and 10% of their diet as erythritol, they drink more water, pee out more water, and lose more calcium, citrate, sodium, potassium, phosphate, and protein in their urine. This study stated the plasma concentrations of these were unchanged but did not show the data, and did not measure plasma osmolality. My suspicion is that this reflects complex adjustments to remove unmetabolized erythritol and balance it with water and solutes in both plasma and urine.
While the mice in the Hazen study had an intact physiology they could use to adjust their plasma osmolality to the injected erythritol, doing so would probably take more than the eight minutes they were given in the study.
Thus, while I cannot say that the osmotic effects were the cause of the clotting propensity observed in the Hazen paper, this should be treated as the leading hypothesis since 1) the Hazen group offered no evidence at all about the mechanism, 2) osmotic stress is a known property of sugar alcohols, and 3) osmotic stress is a known cause of platelet activation.
They could have tested in vitro clotting in blood taken from the human volunteers who consumed the erythritol drink, but they did not report doing so.
This places this finding well below the findings of the same group’s 2017 paper where they did test in vitro clotting of blood taken from volunteers who supplemented choline.
And, by the way, the main physiological function of TMAO is as an osmolyte.
Thus, until shown otherwise, I would assume that the healthy volunteers who consumed erythritol would not exhibit increased clotting as a result of adjustments made to the osmolality of their plasma to accommodate the relative impermeability of erythritol across membranes.
Misleading Language
The Hazen paper uses misleading language to overstate their indictment of “keto” foods.
“One of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and ‘keto’-related foods, erythritol, was among the very top MACE-associated candidate molecules identified.”
Why single out the contribution of keto-related foods in this way when they did not even try to measure dietary intake of erythritol?
They do note that it is hard to quantify erythritol intake because disclosure of erythritol concentrations is not mandated, but they did not report trying to do so, or trying to use any indirect measure such as consumption of keto snacks.
While they do not say outright that they teased out erythritol from poor metabolic health, they seem to imply it with this paragraph:
Across the physiologically relevant concentration range observed in fasting plasma samples, erythritol dose-dependently enhanced platelet aggregation in PRP (Fig. 3a). In contrast, no effect on platelet aggregation responses was observed with either glucose, the most common polyol, or 1,5-anhydroglucitol (AHG), a well-established polyol surrogate of glycemic control (Extended Data Figs. 5 and 6a). Incidentally, we note that 1,5-AHG was negatively associated with cardiovascular event risks in our initial untargeted metabolomics studies (discovery cohort; Extended Data Fig. 1) and in prior reports from large epidemiological studies.
That 1,5-anhydroglucitol (1,5-AHG) did not aggravate thrombosis is easily explained by its ability to equilibrate across membranes as noted above.
That its association with major cardiovascular adverse events is inverse to what one would expect is a little more puzzling.
1,5-AHG can serve as a marker of glycemic control because it is found in food, yet glucose concentrations over 180 mg/dL can prevent the kidney from reabsorbing it. Thus, low levels indicate recent hyperglycemia over the course of several weeks. However, acutely, glucose competes for entry of 1,5-AHG into cells and raises 1,5-AHG for up to two hours. So, presumably fasting levels are inversely correlated to hyperglycemia while postprandial levels are positively correlated to hyperglycemia.
The Hazen study found that 1,5-AHG was slightly lower in those with major cardiac adverse events than those without them among their discovery cohort, who were stable subjects undergoing elective cardiac catheterization. The protocol doesn’t say anything about fasting, so it is not clear whether this represents more hyperglycemia or less hyperglycemia among these individuals. However, it is notable that all of them needed or wanted cardiac catheterization, so had some heart problem of some sort.
The language seems to push one toward believing that keto snacks contribute to heart disease while uncontrolled diabetes is slightly protective, yet this is an absurd takeaway from what they showed.
The Bottom Line
Plasma erythritol likely serves as a marker of a sloppily activated pentose phosphate pathway indicating NADPH depletion and suboptimal thiamin status. The NADPH depletion is caused by oxidative stress, hyperglycemia activating the polyol pathway, and inflammation and insulin resistance increasing de novo lipogenesis. Several of these, especially de novo lipogenesis and suboptimal thiamin status, could be aggravated by a very high consumption of sugar.
The pro-clotting effects shown in vitro and in mice probably reflect osmotic effects of the erythritol that would not play out in a healthy human whose physiology would allow proper control of osmolality in the blood.
On a scale of 1 to 10, this study puts me at about 0.1 in my concern that erythritol might contribute to cardiovascular disease, which is much lower than my 3.0 concern that TMAO might do so. This ranks substantially toward the bottom of the very many things that have been given some plausible suggestions as contributing to heart disease.
I find it extremely suspicious that they added erythritol to blood they took from volunteers to test the pro-clotting effects and did not report trying the same experiment on the volunteers they fed erythritol, even though they had to take blood from them anyway to show that it was very high in erythritol. I find it likely they did try that and did not get the results they wanted.
I do not use erythritol, but were I ever to consider it, I do not believe this study would influence my decision at all.
Stay Immune Through the Winter
My new 7-page quick guide on how to not get sick this winter, Staying Immune Through the Winter, is free for everyone. All you need is a free or paid subscription to my Substack.
If the box above says “subscribed,” this means you are a Masterpass member and can download the guide here.
If the box says “upgrade to paid,” then you are a free subscriber and your guide is currently sitting in your inbox. Search your mail, spam, and trash for “staying immune through the winter” and make sure anything from Substack is going to primary.
If it allows you to enter your email address, do so, hit “subscribe,” and then find the email from “Chris Masterjohn, PhD” with the subject “Welcome to My Newsletter!” If you can’t find this, try searching for either of those terms and for “Substack.” At the top of the welcome email you will find your downloadable guide to not getting sick.
Join the Next Live Q&A
Have a question for me? Ask it at the next Q&A! Learn more here.
Join the Masterpass
Masterpass members get access to premium content (preview the premium posts here), all my ebook guides for free (see the collection of ebook guides here), monthly live Q&A sessions (see when the next session is here), all my courses for free (see the collection here), and exclusive access to massive discounts (see the specific discounts available by clicking here). Upgrade your subscription to include Masterpass membership with this button:
Learn more about the Masterpass here.
Please Show this Post Some Love
Please like and share this post on the Substack web site. This will help spread it far and wide, as subscriber likes drive the Substack algorithm. Likes from paid subscribers count the most, but likes from free subscribers come in the highest numbers and are the foundation for spreading the content.
Take a Look at the Store
At no extra cost to you, please consider buying products from one of my popular affiliates using these links: Paleovalley, Magic Spoon breakfast cereal, LMNT, Seeking Health, Ancestral Supplements, MASA chips. Find more affiliates here.
For $2.99, you can purchase The Vitamins and Minerals 101 Cliff Notes, a bullet point summary of all the most important things I’ve learned in over 15 years of studying nutrition science.
For $10, you can purchase The Food and Supplement Guide for the Coronavirus, my protocol for prevention and for what to do if you get sick.
For $10, you can purchase Healing From COVID Vaccine Side Effects for yourself or a loved one if dealing with this issue. It also contains an extensive well-referenced scientific review, so you can also use this just to learn more about my research into the COVID vaccines.
For $29.99, you can purchase a copy of my ebook, Testing Nutritional Status: The Ultimate Cheat Sheet, my complete system for managing your nutritional status using dietary analysis, a survey of just under 200 signs and symptoms, and a comprehensive guide to proper interpretation of labwork.











Incredibly well-explained, researched and thought-out explanation, Chris. We are all appreciate of your hard work.
This is great analysis!
Chris, thank you do much for your work!