Disclaimer: I am not a medical doctor and this is not medical advice. My goal is to empower you with information. I will not take a position on whether you should or should not get vaccinated. Please make this decision yourself, consulting sources you trust, including a caring health care professional.
In my last post, I laid out the argument that all-cause mortality is the most important metric to look at for evaluating the risk and reward of COVID vaccines. We saw that the American observational data appears to be hidden and obfuscated by the CDC. The English observational data, by contrast, suggests that among young people the vaccinated might be dying at twice the rate as the unvaccinated, but that this data is hopelessly confounded by grouping everyone between the ages of 10 and 59 together. We also saw that, among those over 60, the vaccinated appeared to have an initial mortality benefit during the COVID wave at the beginning of this year, but their benefit spent the rest of the year nosediving. It has now almost disappeared and may or may not be headed for a net mortality increase in the months to come.
The observational data is all confounded by variations in health status, health-seeking and health care-seeking behavior, diet, lifestyle, and lack of control for the distance of time between vaccination and death.
When dealing with such confounders, we must turn to the clinical trials. Since they are large and randomized, they tend to randomly distribute all known and unknown confounders between groups. So, today, we turn to the six-month results of the Pfizer trial. Was all-cause mortality impacted? As we will see, the differences are not statistically significant, but there is cause for concern that the vaccine could be increasing the risk of lethal heart disease.
The Design of the Trial
44,165 people were randomized to the Pfizer vaccine or a saline placebo, 20,030 received at least one dose of vaccine or placebo, and after some of them dropped out for various reasons, 21,759 received both doses of the vaccine and 21,650 received both doses of the placebo.
It is somewhat misleading to call this the “six-month” results of the Pfizer trial. After a two-month followup, which was really a 6-week followup from being “fully vaccinated” (a median of 2 months following the second dose, which is six weeks after the two-week waiting period to be considered “fully vaccinated”), the emergency use authorization (EUA) was given. The cutoff for the “two-month” data used to grant the EUA was October 9, 2020, while the EUA was given on December 11, allowing an additional two months of followup.
Once the EUA was given, the trial participants were given the option to learn which group they were in. If they were in the placebo group, they were given the option to be vaccinated.
The safety data were collected through March 13, 2021, which is four months after the “two-month” cutoff in October, so technically six months. Since unblinding started in December, however, most subjects had fewer than six months of blinded followup. Specifically, a slight majority (51%) had 4-6 months of blinded followup, while about 7% had more than six months of blinded followup and 42% had less than four months of blinded followup.
As such this should be considered the “up to roughly four to six months followup” study.
There will be no further blinded followup because the unblinding has already happened and the placebo group has already been vaccinated. So, we will never have more than “up to roughly four to six months followup” under blinded placebo-controlled conditions for the Pfizer vaccine.
What the Trial Paper Reported
During the blinded phase, there were 29 deaths. 15 people died in the vaccine group and 14 died in the placebo group.
After unblinding, 3 more people from the original vaccine group died, and two placebo group participants died after they, too, had been vaccinated.
Two additional deaths occurred in HIV-positive individuals and these were excluded from the analysis because they intended to report on HIV-positive subjects separately. One occurred in the vaccine group and one in the placebo group.
“None of these deaths were considered to be related to BNT162b2 [the Pfizer vaccine] by the investigators, ” they wrote. “Causes of death were balanced between BNT162b2 and placebo groups.”
What the FDA Reported When Approving COMIRNATY
On November 8 of this year, the FDA released a substantially redacted version of its basis for approving the COMIRNATY version of the Pfizer vaccine on August 23.
According to this document, there were a total of 38 deaths, 21 in the vaccine group and 17 in the placebo group.
Reconciling the Clinical Trial Report With the FDA Approval Basis
It is unclear why there are more deaths in the FDA document than in the trial report.
Both of these documents say their data goes through March 13, 2021. The trial paper was released as a preprint on July 18, 2021, while the initial application for FDA approval was submitted on May 18, two months earlier. The final editing of the FDA document appears to be somewhere between August 20 (a date referred to as in the past several times in the document) and August 23, the date of the approval. The clinical trial paper was published in the New England Journal of Medicine on September 15.
No matter how you slice it, the FDA document is earlier than the clinical trial paper, so the FDA data can't be “more up to date.” Given that they were all written months after the March 13 data cutoff, however, it does not seem possible that either document is more up to date than the other.
If we include the two deaths in HIV-positive people, we arrive at 31 deaths, 16 vaccine and 15 placebo.
If we count all five deaths during the unblinded period as vaccinated deaths (since everyone who died was vaccinated), we arrive at 36 deaths, 21 vaccine and 15 placebo.
It is not clear at all how the FDA arrived at two additional deaths in the placebo group.
The only thing I can think of is that the FDA was not sure whether to count the two deaths in the vaccinated placebo group participants as vaccinated or placebo, so counted them as both.
How Should We Count the Two Deaths in the Vaccinated Placebo Participants?
Since all deaths after unblinding occurred in vaccinated individuals, but three were randomized to receive the vaccine and two were vaccinated after unblinding, I believe the best way to count these deaths would be as follows:
Multiply the number of people by the number of months to arrive at the number of person-months spent in the placebo or vaccine group.
If someone was vaccinated after four months in the placebo group and died one month later, they should contribute four person-months to the placebo group and their death should not be counted in that group; they should contribute one person-month to the vaccine group and their death should be counted in that group.
The analysis would then focus on the number of deaths per person-month in each group.
This would make the deaths look substantially worse for the vaccinated group, because the two deaths among vaccinated placebo group participants would only contribute a maximum of one or two person-months to the group each, but would each contribute their entire death to that group. By contrast, the other deaths in the vaccinated group were contributed by people who spent up to four to six months in that group.
Unfortunately, we do not have the dates at which those people were vacccinated. Therefore, the best solution is to simply count them in the vaccine group.
The apparent solution in the FDA approval document is obfuscatory. A proper analysis would weight these two deaths more heavily in the vaccine group than the other deaths in that group, while the FDA document seems to have neutralized these two deaths by adding them to both groups.
Is the Increase in Mortality Statistically Significant?
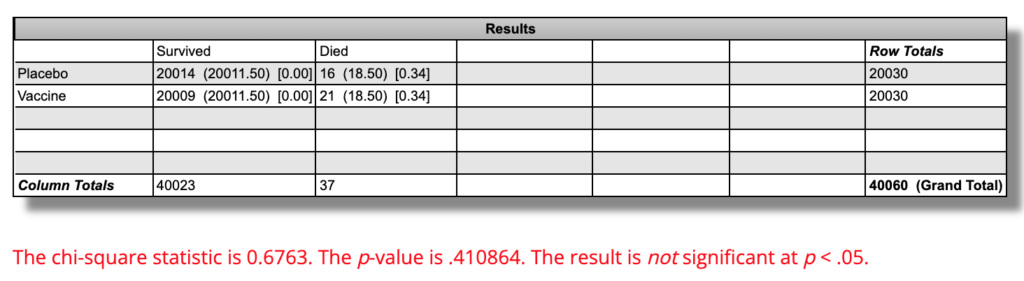
If we use as our final count 36 deaths, 21 vaccine and 16 placebo, we arrive at the following for statistics.
Since two deaths in each group occurred between the first and second dose, and since it is plausible that one dose could have some effect on mortality for better or worse, I am using the number of people in each group who received at least one dose of vaccine or placebo: 20,030.
21 out of 20,030 people in the vaccine group died, which is a mortality rate of 0.105% per “six months” of followup. 16 out of 20,030 people in the placebo group died, which is a mortality rate of 0.0799%.
On an absolute basis, there were 0.025 percentage points more death over “six months” in the vaccine group than in the placebo group. On a relative basis, the “six-month” mortality rate was 31.2% higher in the vaccine group.
Using this calculator, the difference in mortality rates is P=0.41 and is not statistically significant:
This means that if the vaccine had no effect at all on all-cause mortality, and we repeated a trial like this 100 times, 41 of them would show a difference this large or larger.
A Thought Experiment: What If the Trial Had Been Longer?
With that said, a frequent criticism of these clinical trials is that they are not long enough.
If these mortality rates continued similarly into the future, how long would the trial have had to have been to show statistical significance?
By successively testing 6-month intervals where the rates of death are held constant in each group, I find that significance is approached (P=0.65) at 2.5 years, and reaches significance at the 3-year mark:
Conclusion About All-Cause Mortality
The Pfizer trial cannot be used to say that the vaccine increases all-cause mortality, because the increased mortality is not statistically significant and longer followup or a larger trial could look different.
However, the Pfizer trial is consistent with the possibility that the vaccine causes a 31.2% increased risk of mortality, and the fact that it would take three years to show statistical significance if the rates remained constant underscores that these trials were not long enough. It is completely reasonable for someone to want to wait longer to see how the data plays out over time.
Moreover, if there is any mortality-increasing effect of the vaccine, it may be compounded by booster shots. The safety of booster shots has not been studied in any large, randomized, controlled trial like this. We will have to rely on our feeble attempts to tease out the true effects from the highly confounded observational data to wrap our minds around the safety of the booster shots.
Does the Pfizer Shot Increase Heart Disease Deaths?
The specific causes of death are given in Supplementary Table 4, which is on page 11/12 of the Supplementary Appendix:
This data does not include the five vaccinated deaths that occurred after unblinding.
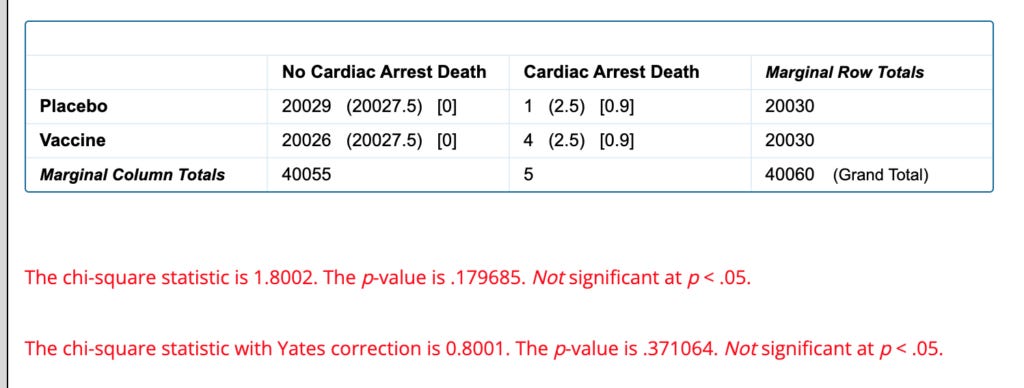
One notable difference is that there are 4 cardiac arrest deaths in the vaccine group and only 1 in the placebo group.
Because there are so few events this requires a correction to the statistical test that makes this wildly insignificant (P=0.8):
This type of analysis is also highly vulnerable to bias, because we are cherry picking the endpoint that looks most different, and we could easily ignore that there were 2 myocardial infarctions in the placebo group and none in the vaccine group.
To reduce bias and to get more events so as to make the statistical tests work better, I believe we should group any type of cardiac disease into one composite endpoint. To do this, I'm grouping aortic rupture, arteriosclerosis, cardiac arrest, cardiac failure congestive, cardiorespiratory arrest, hypertensive heart disease, and myocardial infarction.
There are 5 such deaths in the placebo group and 9 in the vaccine group.
Notably, these four excess cardiac deaths in the vaccine group account for 80% of the total excess deaths in that group.
This still is not statistically significant, but it's a lot closer at P=0.28:
To repeat our thought experiment, how long would the trial had to have been to detect an increase in cardiac mortality of this magnitude if the rates stayed the same over time?
Successively running tests at six-month intervals shows this approaches significance (P=0.064) at the 1.5-year mark and becomes significant at the 2-year mark:
The actual rates of cardiac mortality were 5 out of 20,030 (0.025%) in the placebo group and 9 out of 20,030 (0.045%) in the vaccine group in “six months” of followup. The absolute “six-month” cardiac mortality is 0.01997 percentage points higher in the vaccine group. The relative risk of cardiac mortality over “six months” is 80% higher risk, or 1.8 times the risk, in the vaccine group. This difference is not statistically significant (P=0.28).
In our thought experiment, if the rates stayed the same over time, and the trial lasted two years, this 80% increase in risk would manifest as 20 cardiac deaths (0.0999%) in the placebo group and 36 (0.1797%) in the vaccine group, for 0.0799 greater percentage points of cardiac mortality in the vaccine group in absolute terms. This would be statistically significant at P=0.032.
This, again, underscores the need for longer trials and the reasonableness of wanting to wait to see how the data plays out over time.
It is quite possible that, if the five vaccinated deaths that occurred after unblinding were partly or largely cardiac deaths, it would take less than two years to see significance.
Is Vaccine-Induced Cardiac Death Biologically Plausible?
The American Heart Association journal Circulation recently published a conference abstract claiming that among 566 patients with consecutive measurements before and after mRNA vaccination, inflammatory markers predictive of 5-year acute coronary syndrome risk increased to a magnitude consistent with a 2.3-fold elevation of 5-year risk. This persisted for at least 2.5 months as of the abstract's publication.
Spike protein freely circulates in humans up to 28 days after the Moderna vaccine, and so presumably does so after Pfizer as these are both mRNA vaccines.
The spike protein alone is sufficient to cause vascular endothelial dysfunction in mice.
While some may point out that getting the virus would do the same thing, that's only true in severe cases. For example, viral material showing up in blood was found in 27% of hospitalized cases and 13% of diagnosed outpatients, and so is probably even lower in those who become infected without ever being diagnosed. By contrast, intramuscular injection essentially guarantees circulating spike protein.
This is not meant to be a comprehensive mechanistic case for how the Pfizer vaccine could cause cardiac death. Rather, it is meant as a sampling of studies that are, just by themselves, clear justifications for considering this biologically plausible.
The Bottom Line
While the relative risk of all-cause mortality was 31.2% higher among vaccinated individuals in the Pfizer trial, this was not statistically significant. The trial would have had to have been three years long to make a difference of that magnitude significant, assuming the rates stayed the same over time.
While the relative risk of cardiac mortality was 80% higher among individuals randomly allocated to the vaccine arm of the Pfizer trial during the blinded period, this was not statistically significant. The trial would have had to have been two years long to make a difference of that magnitude significant, assuming the rates stayed the same over time.
Given the complete lack of clarity around the all-cause mortality in the observational data, the precautionary principle calls for taking possible risks found in the trial seriously, even when they aren't statistically significant. It would be wrong to say the Pfizer trial showed an 80% increased risk of cardiac mortality, but it would be wrong to dismiss this just because it isn't statistically significant. Indeed, had the Pfizer trial met the main criticism levied at it by COVID vaccine skeptics by being longer and not vaccinating the placebo group, this 80% increase in cardiac mortality may have persisted and become statistically significant by the two-year mark.
We still don't know how much of the trial was fraudulent. We know that one regional director provided evidence that a contractor was falsifying data, unblinding patients early, and not following up on adverse effects, that Pfizer knew this and went on to use the contractor for four more COVID vaccine trials, and that FDA knew this, turned a blind eye, didn't include the contractor in their site audit, and approved the COMIRNATY version of the Pfizer vaccine. We also know the FDA is asking to have 55 years to fully release the 329,000 pages of documentation used in that approval.
These facts should raise serious suspicions that this trial is full of fraudulent data.
This justifies a heavier dose of skepticism toward the Pfizer vaccine than we would have when taking the trial data at face value.
While the trial does not show definitively that the Pfizer vaccine increases all-cause or cardiac mortality, it is consistent with this possibility. Therefore:
All cases of cardiac mortality that occur after mRNA vaccines should be considered possibly related to the vaccine and reported to VAERS.
For any epidemiological trends suggesting increased cardiac mortality, mRNA vaccines should be considered a possible contributor.
Notably, the age range in the Pfizer trial was 16-89, and the median age was 51. The trial results seem consistent with one interpretation of the observational data suggesting vaccines may increase all-cause mortality in those under 60 while having a short-lived protective effect in older people.
The lack of clarity over this issue underscores that it is imperative for every individual to have the right to choose whether to be vaccinated, free of any type of employment or legal penalty, and free of any social pressure.
Please Show This Post Some Love
Let me know what you think in the comments! And please like the post if you found it valuable, and share the post with others if you believe they too would find it valuable.
Join the Next Live Q&A
Have a question for me? Ask it at the next Q&A! Learn more here.
Subscribe
Subscribe or upgrade your subscription here.
Join the Masterpass
Masterpass members get access to premium content (preview the premium posts here), all my ebook guides for free (see the collection of ebook guides here), monthly live Q&A sessions (see when the next session is here), all my courses for free (see the collection here), and exclusive access to massive discounts (see the specific discounts available by clicking here). Upgrade your subscription to include Masterpass membership with this link.
Learn more about the Masterpass here.
Take a Look at the Store
At no extra cost to you, please consider buying products from one of my popular affiliates using these links: Paleovalley, Magic Spoon breakfast cereal, LMNT, Seeking Health, Ancestral Supplements. Find more affiliates here.
For $2.99, you can purchase The Vitamins and Minerals 101 Cliff Notes, a bullet point summary of all the most important things I’ve learned in over 15 years of studying nutrition science.
For $10, you can purchase The Food and Supplement Guide for the Coronavirus, my protocol for prevention and for what to do if you get sick.
For $29.99, you can purchase a copy of my ebook, Testing Nutritional Status: The Ultimate Cheat Sheet, my complete system for managing your nutritional status using dietary analysis, a survey of just under 200 signs and symptoms, and a comprehensive guide to proper interpretation of labwork.








Thank you for this analysis - the most thorough and balanced review I've seen!
Have you analyzed the serious and adverse reactions between the placebo and vaccinated groups? I'd be curious to know if the results were statistically significant.
Taking my time going through these well researched and presented data Chris. Thank you! I will share the link to your substack. I had the J&J back in April of 2021. Thought it was the lesser of the 3 evils. No issues afterwards and am trying to avoid a booster to go to Italy this summer. I'm 54 fit, metabolically heathly and topped off on my supplements. No Covid infection that I know of and I work in the hospital (pathology)