CoQ10 deficiency is largely a sulfur toxicity problem, and understanding this can explain a variety of anomalies, such as why someone might get anxiety the day after eating meat, or why taurine might protect against manganese toxicity.
As I covered in Does CoQ10 Deserve a Spot on Your Longevity Plan?, CoQ10 is needed to clear hydrogen sulfide.
Hydrogen sulfide is a necessary “gasotransmitter” involved in vasodilation and placental function, among other roles, and is healthy within normal levels.
However, it is also a poison at high concentrations. As I covered in Methylene Blue: Biohacker's delight, or playing with fire?, hydrogen sulfide displaces oxygen in complex IV or the respiratory chain, preventing the use of oxygen to convert food energy to ATP.
Experiments in mice with CoQ10 synthesis defects show that CoQ10 supplementation rescues the kidney damage that would otherwise occur. It does not fix the deficit in the CoQ10-dependent transport of electrons from complexes I and II of the respiratory chain to complex III. It does fix the defective clearance of hydrogen sulfide.
In human cells with CoQ10 synthesis defects from the same study, CoQ10 protected against reactive oxygen species, but suppressing the enzyme that uses CoQ10 to clear hydrogen sulfide abolished this effect. This shows that the reactive oxygen species were coming from poor hydrogen sulfide clearance.
While this does not mean that CoQ10’s role in the respiratory chain is not important, it is compelling evidence that the worst effects of CoQ10 deficiency are from dysregulated sulfur metabolism, not from impairment of the respiratory chain.
Several seeming paradoxes in this study and others emerge:
When CoQ10 clears hydrogen sulfide, it takes electrons away from the sulfur and moves them to complex III respiratory chain, then passes the sulfur onto glutathione. Glutathione then has the sulfur removed as sulfite by the next enzyme in the pathway. Then, the sulfite is delivered to the molybdenum-dependent sulfite oxidase enzyme, which takes more electrons from the sulfur, delivers them to cytochrome C of the respiratory chain, which then delivers them to complex IV, and the sulfur is released as the highly useful sulfate.
You would think that CoQ10 would increase sulfite and decrease glutathione and that its deficiency would reverse these two changes. This is because CoQ10 helps attach the sulfur to glutathione, potentially trapping it, and facilitates the eventual production of sulfite by the next enzyme. However, CoQ10 deficiency decreased glutathione in the mouse study and CoQ10 supplementation restored glutathione to normal.
In humans with deficiencies of the next enzyme in the pathway, which generates sulfite, urinary sulfite is increased, not decreased.
This can be explained as follows:
1) hydrogen sulfide inhibition of complex IV generates superoxide in the respiratory chain, which becomes hydrogen peroxide,
2) hydrogen sulfide reduces ferric iron to ferrous iron, which makes it release from storage in ferritin,
3) this increases Fenton reactions between free iron and hydrogen peroxide, which generate more dangerous reactive oxygen species like the hydroxyl radical,
4) all of this deplete glutathione,
5) since a major purpose of the trans-sulfuration pathway is to provide enough cysteine to make glutathione, glutathione depletion hyperactivates the trans-sulfuration pathway, leading to more cysteine availability, the excess of which is catabolized to sulfite by alternative reactions that do not produce hydrogen sulfide and therefore do not require CoQ10.
Thus, the effect of hydrogen sulfide toxicity is to deplete glutathione and increase sulfite. The sulfite is then available to react with cystine to form S-sulfocysteine, which is a neurotoxin that activates NMDA receptors, ramping up an excited state of the nervous system. The sulfite is then also available to tank testosterone, atrophy testicles, and destroy B vitamins.
In the comments of Iron Overload: Forget What You Thought You Knew, Xavier wrote:
Very interesting, thank you ! I am heterozygous for the C282Y variant. I checked because I used to have all symptoms of iron overload (I still have but milder). I donated my blood a few times but it did not really fix my health. Now, after reading this, it might be because of the manganese (my liver enzymes are not great, which I always found weird because I have a really healthy life).
The one time I felt super great was after a 10 day fast + phlebotomy, but it lasted only 2-3 weeks. It might be because that time the iron loss was not compensated by the manganese intake (because of the fast).
The one think it does not explain, however, is why i tend to feel worse the day after a large meat intake.
Thanks again, only you go into that kind of detail that can change someone life.
As I pointed out in that article, iron overload genes predispose to manganese overload. If he doesn’t feel any better from blood donation, either iron is not his problem, or he has at least one other problem, such as manganese, and he has to fix both to feel better.
As I also pointed out in that article, donating blood while eating a high-manganese diet could worsen manganese overload.
As I covered in Manganese Toxicity Is a CoQ10 Deficiency, interference with the synthesis of CoQ10 is a major mechanism of manganese toxicity, and might be the only or central mechanism.
So, if you are predisposed to iron and manganese overload, clear out the iron overload with blood donation, and continue eating a high-manganese diet, manganese overload will become the primary problem, and this will make CoQ10 synthesis impairment a major problem. That then makes sulfur toxicity a major problem.
Serum sulfate can be used as a proxy for the flux through the sulfite oxidase reaction, and in humans it has a diurnal rhythm that peaks at night:
Hours are plotted on the horizontal axis, with the last point being after an overnight fast.
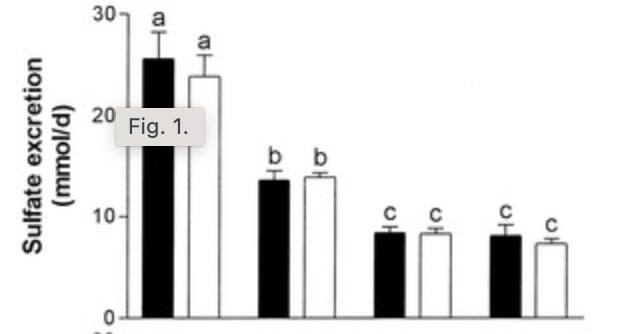
This graph shows 24-hour sulfate excretion on one day where human subjects were habituated to 1.5 grams per kilogram bodyweight protein, followed by several days of habituating to 0.39 grams per kilogram bodyweight protein:
If you continue to fast after waking up, sulfate levels decline. But if you eat 75 grams of carbs, they decline much faster:
This reflects the inhibition of cysteine catabolism by carbohydrate that I discussed in Will Longevity Diets Wreck Your Hormones?
I in general am skeptical of “diurnal rhythms” that do not experimentally manipulate eating patterns. Sure sulfate is higher at night, but why? To me it is rather obvious: you have eaten an average of three protein-containing meals and you’ve been running trans-sulfuration all day long.
Yes, it will be lower in the morning, usually. But is that the case if you protein-bomb the night before?
I wouldn’t be so sure.
Further, there are lots of other interacting things determining how you feel. If you relax properly at night, your cortisol bottoms out, your adrenals calm down, and your parasympathetic nervous system kicks in. If you wake up with S-sulfocysteine accumulating and then get a morning sunshine cortisol spike and have two cups of coffee and start working, then the cumulative neurostimulant effect of the S-sulfocysteine starts really impacting how you feel.
Or maybe you just feel less vital. That could be the testosterone-tanking effect of sulfite.
Or it may be the destruction of B vitamins.
Regardless, more meat and less carbs means more trans-sulfuration. If you’re missing CoQ10 because of manganese overload, that means more hydrogen sulfide, sulfite, and S-sulfocysteine.
Rahat Iram asked on Facebook, isn’t manganese toxicity about taurine?
Yes, there is evidence that taurine reverses the cognitive impairments in rats and reverses the mitochondrial damage in mice.
This fits perfectly into the model: taurine suppresses the trans-sulfuration pathway, as does glutathione, such that the pathway turns on only when we need taurine or glutathione. This will prevent the overproduction of cysteine, and thus the overproduction of hydrogen sulfide and sulfite.
Taurine is also an inhibitory neurotransmitter that will counteract the effects of S-sulfocysteine.
Thus, those with iron overload genes should be very concerned about manganese overload, CoQ10 deficiency, and sulfur toxicity.
So should everyone who benefits from CoQ10. As I explained in Does CoQ10 Deserve a Spot on Your Longevity Plan?, benefitting from CoQ10 is a sure sign that you have a genetic or nutritional reason for that benefit, and this means managing your CoQ10 status is critical to preventing sulfur toxicity.






What an incredibly well-rounded article! The complexity of our biology is both fascinating and scary - especially when you notice things going out of balance. Thank you for the great work and, as always, looking forward to learning more.
Terrific info. Does coq10 supplementation run a risk of depleting riboflavin?