Iron Overload: Forget What You Thought You Knew
Everything we think we know about iron status starts to crumble when we step back and appreciate the role of manganese.
Iron overload causes liver damage, diabetes, heart problems and brown, bronze, red, or gray discoloration of the skin when it gets severe.
More moderate iron overload causes fatigue, joint pain, depression and mood swings, hair loss, chest pain, dizziness, impaired sexual function, menstrual problems, and abdominal pain.
Iron overload raises cholesterol, increases the risk of Alzheimer’s and Parkinson’s, and it causes general wear and tear on tissues that accelerates the aging process.
This has long been known in the medical communities and the health and wellness spaces.
Yet, everything we think we know about iron status starts to crumble when we step back and appreciate the role of manganese.
Manganese overload causes headaches, irritability, insomnia, depression, and eventually loss of balance, problems walking normally, and Parkinson signs such as rigidity and tremor.
About 9% of people globally are at least heterozygous for a genetic impairment in iron handling, usually in the HFE gene, and usually either the more severe C282Y variant or the less severe H63D variant. This predisposes men to iron overload, and women to an iron overload that tends to be delayed by decades as a result of losing iron during menstruation.
Conventional medicine looks only at the one in 200 to 500 people with the diagnosable criteria of hemochromatosis.
Functional medicine looks at the 9% and tries to optimize iron status.
Functional medicine is correct in this point, because moderate iron overload can cause fatigue, high cholesterol, diabetes, and generally accelerated aging.
But here is where everything falls apart:
These impairments don’t simply cause iron overload, they cause manganese overload too.
You can’t just donate blood. 90% of your iron is stored in your blood, but most of your manganese is stored in your liver.
When you donate blood, your iron status declines, which makes you more vulnerable to manganese toxicity.
If you restrict iron in your food, you have less competition for absorption and transport and your iron status declines which makes you more vulnerable to manganese toxicity.
If you eat less meat and more veggies to avoid heme iron, you will drown yourself in potentially toxic manganese.
Let’s not even get into the wildly dangerous topic of chelation, which can give you deficiencies of all kinds of minerals. The big problem here is that everything you thought you should do to prevent iron overload makes you more vulnerable to manganese toxicity, and the genetic basis for the concern about iron is equally a genetic basis for concern about manganese.
So what do we do about it?
That is the topic of this article.
The Short Answer
The short answer is that if you have a genetic predisposition to iron overload, you should restrict dietary manganese to no more than two milligrams per day. When actively donating blood, be especially strict about this limitation and consider reducing intake to one milligram per day for the four weeks after donation. If predisposed to iron overload, make sure your iron intake is always at least four times your manganese intake. Restricting manganese can be accomplished by reducing plant foods and focusing on source of carbohydrate that are low in manganese. Examples include milk, potatoes, apples, pears, oranges, litchis, plantains, bananas, grapefruit, cherries, peaches, raisins, dates, papaya, navy beans, cranberries, and pomegranates.
You can use the MyFoodData Nutrient Ratio Tool to brainstorm additional foods that fit the above criteria, and use Cronometer with my Custom Nutrient Targets to assess and tweak your diet.
The degree to which you need to worry about this depends on the degree to which this genetic predisposition is a major driver of your health. While determining that is beyond the scope of this article, the best tool to get started assessing this is whole genome sequencing. This will allow you to see if you have any of the 215 variants across the 5 hemochromatosis-related genes — 38 in HFE, 55 in HFE2, 13 in HAMP, 49 in TFR2, and 60 in SLC40A1 — rather than only knowing if you have one of the two most common variants in the HFE gene. Pair this by running the Comprehensive Nutritional Screening and using the algorithm in the Cheat Sheet to determine iron status. Markers indicating iron overload prior to any blood donation along with agreement from genetic variants should be used to infer manganese overload without respect to blood manganese.
Is Hemochromatosis Really About Manganese Toxicity?
In 1967, medical researchers from the Divisions of Medicine and Nuclear Medicine at the Wa!ter Reed Army Institute of Research examined the manganese contents of livers from people dying of hemochromatosis and people dying of myocardial infarction. The livers of the hemochromatosis patients had twice as much manganese.
The reason for their inquiry was as follows:
Bleeding and iron deficiency both upregulate manganese absorption in rats, suggesting that manganese and iron share transport mechanisms. This means that if hemochromatosis causes iron overload, it should also cause manganese overload.
Iron overloading does not cause liver cirrhosis in rats and iron infusions do not cause liver cirrhosis in humans, but injecting rats with manganese does cause liver cirrhosis.
Humans with hemochromatosis get liver cirrhosis. Thus, maybe they get liver cirrhosis because they have manganese overload.
Their results showing that liver manganese was twice as high in hemochromatosis livers than controls was consistent with this hypothesis.
Rats do develop fibrosis from iron overload, which is the laying down of scar tissue in the liver. However, cirrhosis refers to an advanced stage of scarring that is considered irreversible, and where the scarring profoundly impairs the normal function of the liver.
Even by 1994, the effects of iron overload on rat liver were considered “pre-cirrhotic,” although their livers can be made cirrhotic by toxins such as carbon tetrachloride, and, unlike rats, gerbils develop cirrhosis in response to iron overload just fine.
Mutations in SLC30A10, a transporter that removes excess manganese from cells, leads to normal or low iron, and accumulation of toxic levels of manganese in multiple tissues, including the liver and brain. The liver complications in humans with these mutations lead to cirrhosis.
While it’s probably the case that iron overload is sufficient to cause cirrhosis in humans, it does not occur without manganese overload, and the cirrhosis of SLC30A10 deficiency shows proof of principle that manganese overload can cause cirrhosis in humans without iron overload. This offers at least partial support to the original supposition that the manganese accumulation in hemochromatosis is “the cause” of the cirrhosis of hemochromatosis, and we can at least say that it is “a contributor” to it.
Iron and Manganese Share Transport Mechanisms
In 1971, it was shown that people with lower iron stores have higher manganese absorption. Experiments in rats suggested this was because they share a transport system that is upregulated in iron deficiency, and that direct competition between the two provided at the same time is mostly irrelevant except at very high doses.
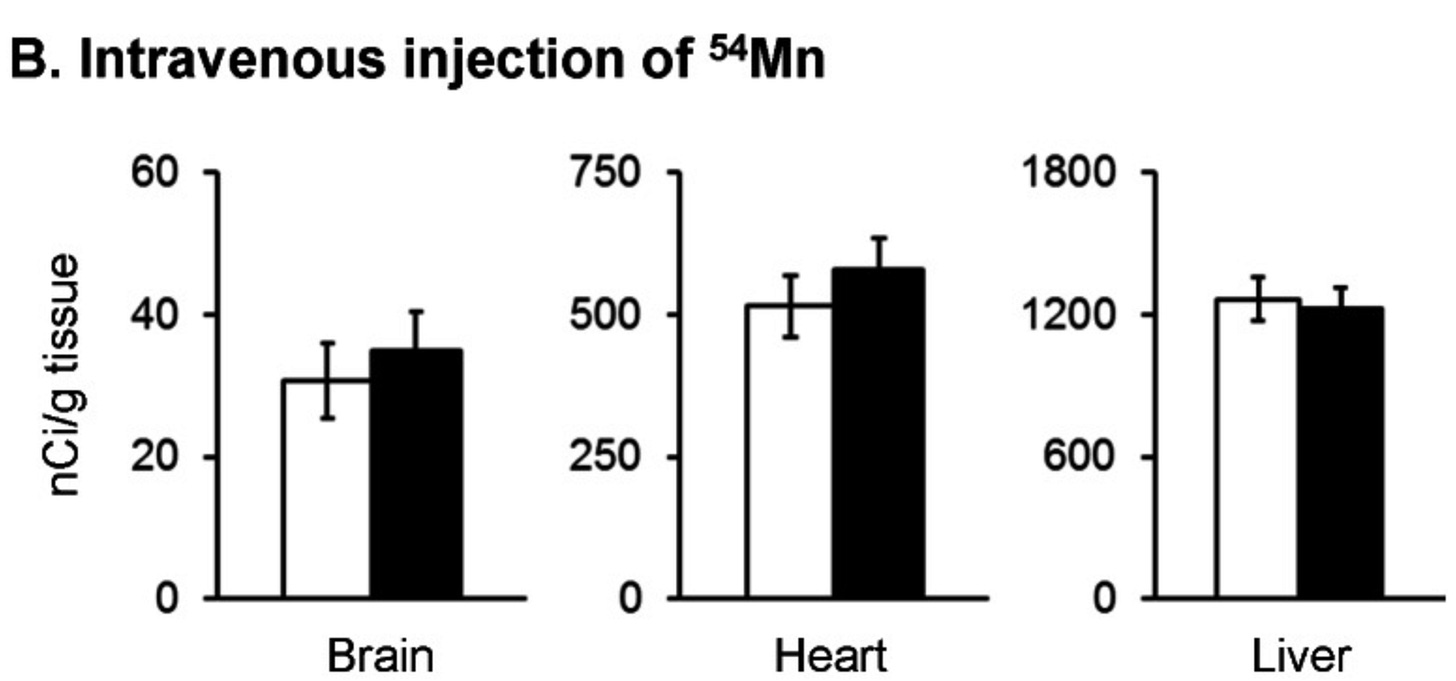
Fast-forward to 2013, when researchers from Northeastern University and Harvard School of Public Health provided remarkable evidence in mice that loss of the HFE gene, the major cause of hereditary hemochromatosis, causes increased absorption of manganese from the gut and the nose.
When labeled manganese was flushed into their stomachs, appearance in the blood increased by 56%:
When iron was injected into the blood, loss of HFE biased its deposition into liver and heart. This was not true for manganese, suggesting that once the two minerals are in the blood, the pathways involved in their transport are different.
However, visually inspecting the data suggests that there might be an increased deposition in brain and heart that escaped statistical significance:
These researchers never looked at the accumulation of manganese in the liver after oral feeding. When you eat something, the water-soluble components travel from the intestine to the liver via the portal vein before the liver releases them into the general circulation. Since manganese would reach the liver before the blood after eating it, the increase in blood manganese probably underestimates the increase in liver manganese.
They generated some remarkable findings when instilling the manganese into the noses of the mice.
First, much more manganese made it into the blood of all the mice after intranasal administration than after intragastric administration. This suggests that inhaling manganese is much more dangerous than eating it, since absorption across the gut is much more tightly regulated than absorption into the lungs.
Loss of HFE appeared to direct the manganese into the brain at the expense of the blood:
This seems to conflict with an older study from 2001 that found loss of the gene for beta-2-microglobulin, a protein that interacts with HFE, did not alter manganese balance at all. However, the above study fed a diet with 8-11 times more iron and manganese than the older study, so I think it is possible that the regulatory system needs to be stressed by high intakes in order to show the manganese effect.
HFE mutations seem to impact the distribution of manganese in the brain as well. In mice exposed to toxic levels of manganese by inhalation, loss of HFE makes toxic levels of inhaled manganese less likely to cause impulsivity and more likely to cause anxiety. It causes manganese levels in the cerebellum to be lower, suggesting it might protect against manganese-induced movement disorders. Loss of HFE also protects mice against manganese-induced memory loss. However, things look somewhat different if manganese is fed in the drinking water. In that case, loss of HFE drives protects against motor deficits, as you would expect from the inhalation study, but it increases memory loss, contrary to the inhalation study.
On the other hand, the mouse version of the H63D allele seems to protect mice across the board from inhaled manganese.
Ferroportin is a transporter traditionally identified with export of iron from the intestinal cells into the blood — and thus, iron absorption from food — and from cells that store iron into the blood. Mutations in the ferroportin gene are a less common cause of hemochromatosis alongside mutations in HFE. In 2019, researchers from the University of Michigan showed that disease-causing mutations in the ferroportin gene prevent cells from exporting manganese, and make them vulnerable to dying from oxidative stress when exposed to concentrations of manganese that are harmless to normal cells.
This study also showed that there was a slight tendency for increasing manganese to lead to lower cellular iron, suggesting a competition for uptake, a displacement effect, or an upregulation of the common export pathway.
In mice, loss of ferroportin biases manganese away from the bone, muscle, kidney, and lung, and into the brain. Dietary iron overload drives manganese out of the liver and into the spleen, and causes oxidative stress from cellullar manganese deficiency that is corrected with manganese supplementation.
Further Evidence in Humans
Hemochromatosis patients have twice as high blood manganese as healthy controls, while Mexican women with at least one copy of a defective iron-related gene have lower blood manganese. If we synthesize this with the animal experiments, the Mexican women probably have their manganese biased toward their livers and brains, while the diagnosed hemochromatosis patients have accumulated iron to such an extent that there is more complex dysregulation that raises the manganese levels in the blood.
In a small population living near a ferromanganese refinery in Marrieta Ohio, possession of a C282Y allele in HFE was associated with 45% greater manganese in hair. This study did not have the statistical power needed to look at homozygotes specifically. Having an H63D allele in HFE was associated with 6% greater manganese in hair, but this was not statistically significant.
What Does This Mean?
There is still a lot to learn about manganese transport. It shares some pathways with zinc, and experiments in human cells suggest that zinc, iron, and manganese share the same way of getting into the intestinal cell, but that only iron and manganese share the same way of getting from the intestinal cell into the rest of the body.
Its role in the liver cirrhosis of hemochromatosis deserves controversy and further research, but our main concern should be neurotoxicity from absorbing too much manganese from food.
What we know is as follows:
The iron transporters DMT1 and ferroportin are responsible for the complete pathway of manganese and none-heme iron absorption from food.
Hepcidin is a hormone that responds primarily to high iron status and mediates decreased absorption of iron from food and increased storage of iron in the protective protein known as ferritin.
Changes in hepcidin will alter manganese transport in largely the same way they alter iron transport.
If you have hemochromatosis-related mutations that predispose you to iron overload, they will proportionally predispose you to manganese overload.
If you donate blood to clear iron, you will not clear your manganese, and your absorption of manganese from food will increase. If you restrict dietary iron, your absorption of manganese from food will increase.
Thus, predisposition to iron overload is a huge reason to limit manganese intake from food.
Since manganese overload runs the risk of displacing iron in cells and in iron-dependent enzymes, it is critical to never restrict iron more than manganese when trying to reduce iron status, as this would make any manganese overload tremendously worse. The estimated average requirements from the National Academy of Medicine are 8 milligrams of iron and 2 milligrams of manganese for adults, so as a rule of thumb I think it is wise for those trying to reduce iron overload to always keep an iron-to-manganese ratio of at least 4.
Since the estimated average requirement for manganese is 2 milligrams, I think this is also a good target to limit manganese to if you have a problem with iron overload.
The data clearly indicate that manganese overload should be inferred on the basis of iron overload.
Blood levels of manganese should not be used for this purpose because manganese overload is likely to be in the liver and brain, not the blood.
Therefore, the algorithm in the Cheat Sheet for iron overload should be used to assume manganese overload, and this should be based on baseline pre-blood donation data. Donating blood will NOT fix manganese overload, but it will change iron markers.
The Bottom Line
Here’s the bottom line:
Use whole genome sequencing to look for relevant mutations in HFE, HFE2, HAMP, TFR2, and SLC40A1.
Run the Comprehensive Nutritional Screening. Use the algorithm in the Cheat Sheet to determine iron status.
In the presence of genetic evidence and blood marker data prior to blood donation, assume the degree of iron overload is reflected proportionally by manganese overload.
To the extent the iron overload predisposition is a major driver of your health, adhere to the limitation of 2 milligrams manganese per day and an iron-to-manganese ratio greater than 4.
Be especially strict in the four weeks after a blood donation and consider limiting manganese to one milligram per day during that period.
Manganese restriction can be accomplished by preferring animal foods over plant foods, not eating mussels, and getting carbohydrates from foods with high carbohydrate-to-manganese ratios, such as milk, potatoes, pears, oranges, litchis, plantains, bananas, grapefruit, cherries, peaches, raisins, dates, apples, papaya, navy beans, cranberries, and pomegranates.
You can use the MyFoodData Nutrient Ratio Tool to brainstorm additional foods that fit the above criteria, and use Cronometer with my Custom Nutrient Targets to assess and tweak your diet.




I have Hemochromatosis. I was diagnosed with it nine years ago. This is interesting! That’s an understatement. I’ll be sharing this with a couple of large groups I’m in with people like me
I am glad to read this article. More people should know.