Cholesterol Chemistry 101
Click on any of the links below for a more in-depth explanation of the terms!
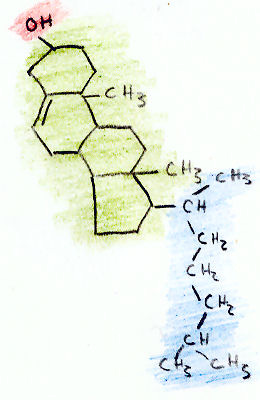
The Structure of Cholesterol
Cholesterol has a molecular formula of C27H45OH. This molecule is composed of three regions (shown in the picture above): a hydrocarbon tail (shown in blue), a ring structure region with 4 hydrocarbon rings (shown in green), and a hydroxyl group (shown in red.).
The hydroxyl (OH) group is polar, which makes it soluble in water. This small 2-atom structure makes cholesterol an alcohol. The alcohol that we drink, ethanol, is a much smaller alcohol that also has a hydroxyl group (C2H5OH).
The 4-ring region of cholesterol is the signature of all steroid hormones (such as testosterone and estrogen). All steroids are made from cholesterol. The rings are called "hydrocarbon" rings because each corner of the ring is composed of a carbon atom, with two hydrogen atoms extending off the ring.
The combination of the steroid ring structure and the hydroxyl (alcohol) group classifies cholesterol as a "sterol." Cholesterol is the animal sterol. Plants only make trace amounts of cholesterol, but make other sterols in larger amounts.
The last region is the hydrocarbon tail. Like the steroid ring region, this region is composed of carbon and hydrogen atoms. Both the ring region and tail region are non-polar, which means they dissolve in fatty and oily substances but will not mix with water.
Because cholesterol contains both a water-soluble region and a fat-soluble region, it is called amphipathic.
Cholesterol, however, is not water-soluble enough to dissolve in the blood. Along with fats and fat-soluble nutrients, therefore, it travels in the blood through lipoproteins such as LDL and HDL.
Need a Chemistry Refresher Course?
If your chemistry skills need brushing up on, simply click on any of the terms for an in-depth Cholesterol Chemistry 101 lesson on that term. Or, simply start at the first lesson, and work your way through until you graduate!
You can also share this article, or leave a comment below.