Of the seventy-four atoms in the cholesterol molecule, only two of them form the hydroxyl group. But the hydroxyl group is not only important because it makes cholesterol an alcohol, but becuase it is the only polar part of the molecule. This small polar region is water-soluble. The rest is fat-soluble.
But what does all this mean...?
The Hydroxyl Group is Polar
The hydroxyl group consists of an oxygen atom bound to a hydrogen atom. The picture below shows an oxygen and hydrogen atom that have not formed a bond.
Each of the above atoms isneutral. This means it has neither a positive nor a negative charge. Both atoms each have an equal number of protons (positive charges) and electrons (negative charges) that balance each other out.
Although we can count the number of protons and electrons, the pictures are shown simplistically to cut to the chase, so don't show the individual particles.
Also, electrons whir about the nucleus as if they are a cloud around it, so it is shown that way above with the yellow cloud representing the electrons and the circle representing the nucleus (where the protons are.)
But when the two atoms form a bond, and a hydroxyl group is formed, something changes. Between the two, there arestillan equal number of electrons and protons, so the group is neutral. However, the electrons tend to hang out more on one side than the other, so their negativity concentrates a little more on that side. Naturally, the other side becomes a little more positive.
You can see in the above picture that the electron cloud surrounds the whole hydroxyl group, but it is much more drawn towards the oxygen. Since the group as a whole is still neutral, but just has an uneven distribution of charges, we call these partial charges.
The oxygen in a hydroxyl group is called partially negative, and the hydrogen is called partially positive. Since there is a concentration of opposite types of charge on each end of the group, it is called polar, having positive and negative poles. (Like a magnet or the earth has opposing "north" and "south" poles.)
Water as an Example of a Polar Molecule
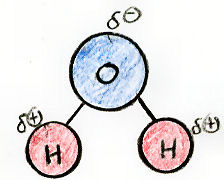
Notice that the hydroxyl group found in cholesterol is also found — twice — in... water!
You can see in the above diagram that since water is composed of two polar bonds, it also has concentrations of charge. The oxygen is partially negative and both hydrogens are partially positive.
I'm sure you've heard the saying "opposites attract." Right? Sometimes that works with people, but it always works with magnets (north and south attract)... and electric charges (positive and negative attract).
Polar Molecules Mix with Water
If you have a glass of water, even when it looks like it's not moving, two things that you can't see are always going on. First, weak bonds are forming between the partially positive hydrogens of one molecule and the partially negative electrons of another molecule. Second, they are constantly being ripped apart because the molecules are always moving around.
Have you ever mixed oil and water together only to find that most of the oil sits on top, and what little cracks the surface of the water is in large droplets that float back up? Yet if you mix, say, sugar, it dissolves and seems to disappear.
The reason is that the sugar contains hydroxyl groups.
The partially positive hydrogens on the sugar are attracted enough to the partially negative oxygens of water, and vice versa, that each sugar molecule has the strength to break through the weak bonds between individual water molecules. The oil has no partial charges (it is non-polar), so it has no attraction to the water's partial charges.
Cholesterol is Amphipathic
Cholesterol has a small, water-soluble polar region that dissolves in water, but nearly the entire cholesterol molecule is non-polar, which will NOT dissolve in water — like oil. This makes cholesterol an example of an amphipathic molecule — part water-soluble, part water-insoluble.
The picture below demonstrates this property.
In the picture above, the water molecules form a "cage" around the polar hydroxyl group. When you dissolve sugar in water, each sugar molecule is bound up in a similar "cage", surrounded by a cluster of water molecules.
But most of the cholesterol molecule has no attraction to the water. If you tried dissolving pure cholesterol in water, like the oil, it would sit at the top. Yet one interesting thing would happen at a microscopic level: all the cholesterol molecules would arrange themselves so that the tiny polar hydroxyl groups were pointing into the water!
As you peruse through the information on this site, you will see that this interesting property is very important to how cholesterol works in your body.
You can read more about the non-polar regions of cholesterol in the next lesson...