Cholesterol contains a region with four hydrocarbon rings. A hydrocarbon ring is a ring of carbon atoms which are each connected to each other. There are usually six carbons but there can be many different numbers of carbons. Two hydrogens extend off each carbon, and thus hang off the edge of the ring.
For simplicity, rings are usually drawn without showing the carbons and hydrogens. It is assumed that at each corner in the ring there is a carbon, and the presence of the hydrogens is also assumed.
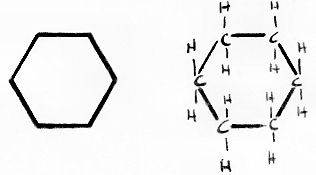
For example, the picture below shows two ways of showing cyclohexane, which is a hydrocarbon ring with six carbons:
In the above picture, the example on the left is how ring structures are typically shown, and is how the ring structures are shown in the picture of cholesterol on the main page of Cholesterol Chemistry 101.
The example on the right is identical to the one on the left, but shows the carbons and hydrogens in detail. Each corner of the ring represents a carbon, and each carbon may be assumed to hold two hydrogens.
Notice that each carbon is bound to four other atoms: two other carbons, and two hydrogens. Each hydrogen is only bound to one atom: a carbon. Carbon always binds to four other atoms, and hydrogen always binds to one atom. So, if another structure is shown protruding off a ring structure, then the carbon it is connected to must be bound to one less hydrogen.
Always assume the number of hydrogens connected to a carbon to be four, minus the number of other atoms it is shown binding to.
Double Bonds in Hydrocarbon Ring Structures
Another way the number of hydrogens bound to a carbon can decrease is if there is a double bond between two carbons. When this occurs in a ring structure, a second line is drawn between the two corners, representing the second bond, as shown on the left below:
The molecule above is benzene, which is the same as cyclohexane but with alternating double bonds instead of single bonds.
The molecule shown on the right is equivalent to the one shown on the left. Because each carbon is involved in a double-bond, there are three, instead of two, bonds for each carbon without involving the hydrogens. Therefore, each carbon can only bind to one hydrogen instead of two.
Cholesterol Contains Hydrocarbon Rings
In the case of cholesterol, there are four hydrocarbon rings. Three of them are six-carbon rings, and one of them is a five-carbon ring. There is a hydroxyl group protruding off one ring, a double bond in one, two protruding CH3 groups in the middle, and a hydrocarbon tail protruding off the end. Each of these structures reduces the number of hydrogens bound to the carbons to which they are attached.
Because hydrocarbon rings involve a bond between carbon and hydrogen, which is non-polar, the hydrocarbon ring portion of cholesterol is fat-soluble and is not soluble in water. This will be explained in more detail in the next lesson...