In Why You Need to Start Juicing Tonight: The Sleep-Anxiety Connection Nobody's Talking About, I covered how juicing romaine lettuce could supply tetrahydrofolate (THF), an important form of folate that is not available as a supplement.
It is not available because the supplement industry either doesn’t see the point or prematurely gave up because THF is extremely unstable, even though there is plenty of inspiration from nature for how to stabilize THF, such as the folate-binding protein present in raw milk or the unidentified factors that make THF and other forms of folate extremely stable in liver.
Here is the biochemistry of why you may need THF and why other forms of folate may hurt you.
WARNING: THIS IS FULL OF SCIENTIFIC DETAIL
Attention all biochemistry nerds: keep reading.
All others: try juicing 100-300 grams of romaine lettuce as a THF supplement if you have a high serine-to-glycine ratio or a high urinary formiminoglutamate on the Genova ION + 40, especially if red blood cell folate is not low on the On the Vibrant America Micronutrient Panel, with these ideally tested as part of the Comprehensive Nutritional Screening.
This is educational in nature and not medical or dietetic advice. See terms for additional and more complete disclaimers.
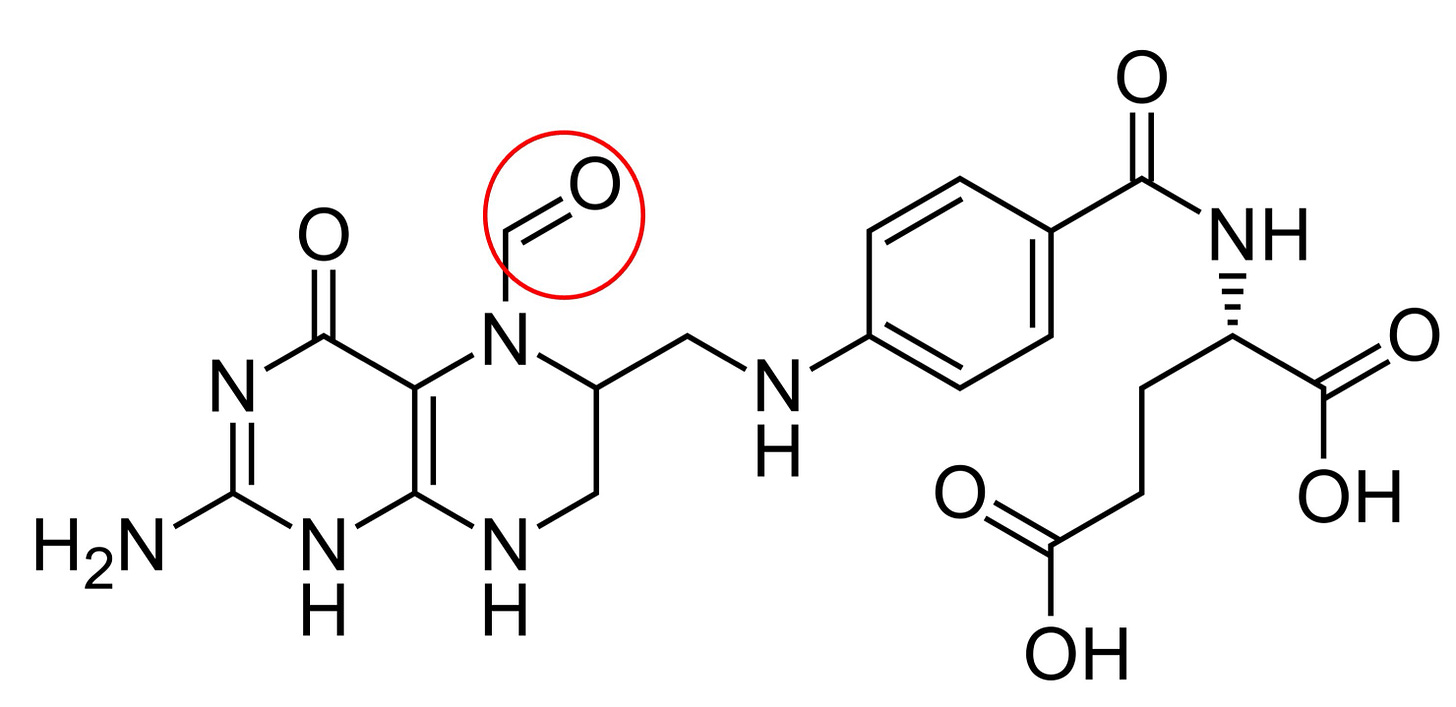
The Structure of Folate
Shown below is folic acid, which does not exist in nutritionally meaningful amounts in nature, and is found in supplements only in synthetic form. It probably does exist in trace amounts in nature, but measuring folate species requires substantial extraction from foods and it could be debatable whether the finding of trace folic acid in plant and animal tissues is an artifact of the extraction (meaning some folate degraded to folic acid during extraction) or whether it is naturally found in trace amounts.
The figure is from the Folic Acid chapter of Modern Nutrition in Health and Disease and it’s a useful figure to show the components of the folic acid molecule and all of its related folates.
The “pterin” on the left comes from the Greek word for “wing” due to the presence of pterin-based pigments such as xanthopterin in the wings of butterflies. Pterins are also present in the molybdenum cofactor (as discussed in The Science Behind the Sulfur Protocol), and in the endogenously synthesized cofactor tetrahydrobiopterin (BH4).
para-Amino-benzoic acid (PABA) is similar to benzene (a hexagonal ring with alternating double bonds), with a carboxyl group on one side (COOH, in this case it is forming a peptide bond with glutamate to the right; this is what makes it an “acid”), and an amino group sticking out on the left (denoted “para” for being directly across from the carboxyl group), making a carbon bridge to the pterin ring.
Folate molecules have variable numbers of the amino acid glutamate attached. When amino acids have their acidic or amino groups joined to other molecules, they are either no longer “amino,” no longer “acids” or are neither, so they are called amino acid “residues.” A glutamate thus becomes a “glutamate” or “glutamyl” (as an adjective) “residue.”
In the diagram, one glutamyl residue is found on the right. The most common number of glutamates inside cells is five (called penta-glutamyl folates). When we digest folate, we break it down to have one or two glutamates, but when we use it we build it back up to a larger number of glutamates, with, once again, five being the most common. The accumulation of multiple glutamates on the end of the folate molecule appears to play a role in trapping it inside cells and stabilizing it on folate-dependent enzymes.
At the nitrogens marked “5” and “10” we can have various “substitutions” (which are more intuitively thought of as “additions”) such as a methyl group, a methylene group, a methenyl group, or a formyl group.
In chemistry, the difference between a corresponding “-ate” and “-ic acid” is that the “-ic acid” has an ionizable hydrogen ion, while the “-ate” has lost it. That is, the “-ate” is the conjugate base of the “-ic acid.”
In folate science, we have a jargonism, which is designed to confuse outsiders to create a scientific priesthood based on knowledge of the pointless deviations from normal language. Thus, in folate science, “folate” or “folates” refer to natural compounds that have extra hydrogens on the inside pterin ring and variable numbers of glutamates on the end, while “folic acid” refers to the absence of these hydrogens and the presence of only a single glutamate on the right.
The interconversion of folic acid with tetrahydrofolate, which is the base molecule for all natural folates, is not an acid-base reaction, but rather a redox reaction, which means additional electrons are added to glue the hydrogens onto the molecule. Thus, the “-ic acid” and “-ate” distinction is a jargonism with no relation at all to the usual use of these suffixes throughout the rest of chemistry.
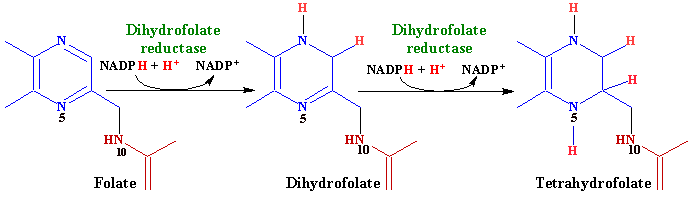
Dihydrofolate and Tetrahydrofolate
The figure below from this site misses the point of the jargonism and “incorrectly” calls the molecule on the left “folate,” whereas those who understand the jargonism would call it “folic acid.” Much of the molecule is abbreviated to draw attention to what is being changed:
The enzyme dihydrofolate reductase (DHFR) will add hydrogens to the top nitrogen of the inside pterin ring, reducing it to dihydrofolate. It will then do the same to the bottom nitrogen of the pterin ring, making tetrahydrofolate (THF). These are supplied by niacin (vitamin B3) in the form of NADPH, which took the electrons from glucose in the pentose phosphate pathway. See my lesson on that pathway here:
If you are not supplementing with synthetic folic acid, this enzyme will mostly be involved in converting dihydrofolate to tetrahydrofolate (THF). These names follow straightforwardly from two or four hydrogens being added to the “folic acid” base molecule.
One-Carbon Substituted Folates
The THF molecule can have various one-carbon groups added to it, which is why the folate cycle and methylation system are often called “one-carbon metabolism.”
The simplest carboxylic acid is formic acid, and has only one carbon:
When it is added to other molecules, it is called a “formyl group.”
When added to the 5 position on THF it forms 5-formyl-THF:
In this case the OH has been removed from the formic acid and the carbonyl (C=O) is attached to the nitrogen.
The formyl group is glued onto the molecule using energy from ATP, while the OH is removed in the formation of free phosphate as the ATP is broken down to ADP and phosphate. (Phosphate needs an extra OH to exist in free form compared to as part of the ATP molecule.)
This could also be performed on the 10-nitrogen to form 10-formyl-THF.
Single carbons that are not attached to oxygen can have variable numbers of hydrogens, depending on what else they are attached to. A methenyl group has one hydrogen, a methylene group has two, a methyl group has three, and if carbon had four it would be a free-floating molecule of methane.
The enzyme MTHFD1 adds a formyl group to the 10-position on THF to form 10-formyl-folate using ATP as just described. It then removes the oxygen to release water and joins the resulting methenyl group to the 5-position, forming a bridge.
This is 5,10-methenyl-THF.
MTHFD1 then performs its third task in using niacin (vitamin B3) in the form of NADPH to add a hydrogen to the carbon, forming 5,10-methylene-THF:
In the diagram, the addition of hydrogens manifests as a removal of double bonds. This is because all carbon atoms have the potential to bind to four atoms, so if they are deficient in hydrogens they form multiple bonds with nearby carbon atoms to reach a total of four.
For simplicity in these figures, all points at the ends of lines, including those within rings, represent carbon atoms, and it is assumed that all empty positions are filled with hydrogens.
MTHFR, an enzyme that depends on riboflavin (vitamin B2) then adds an additional hydrogen using NADPH to form the methyl group of 5-methyl-THF.
This methyl group can then be donated in the methylation cycle, generating THF, which would allow the process to be repeated all over again.
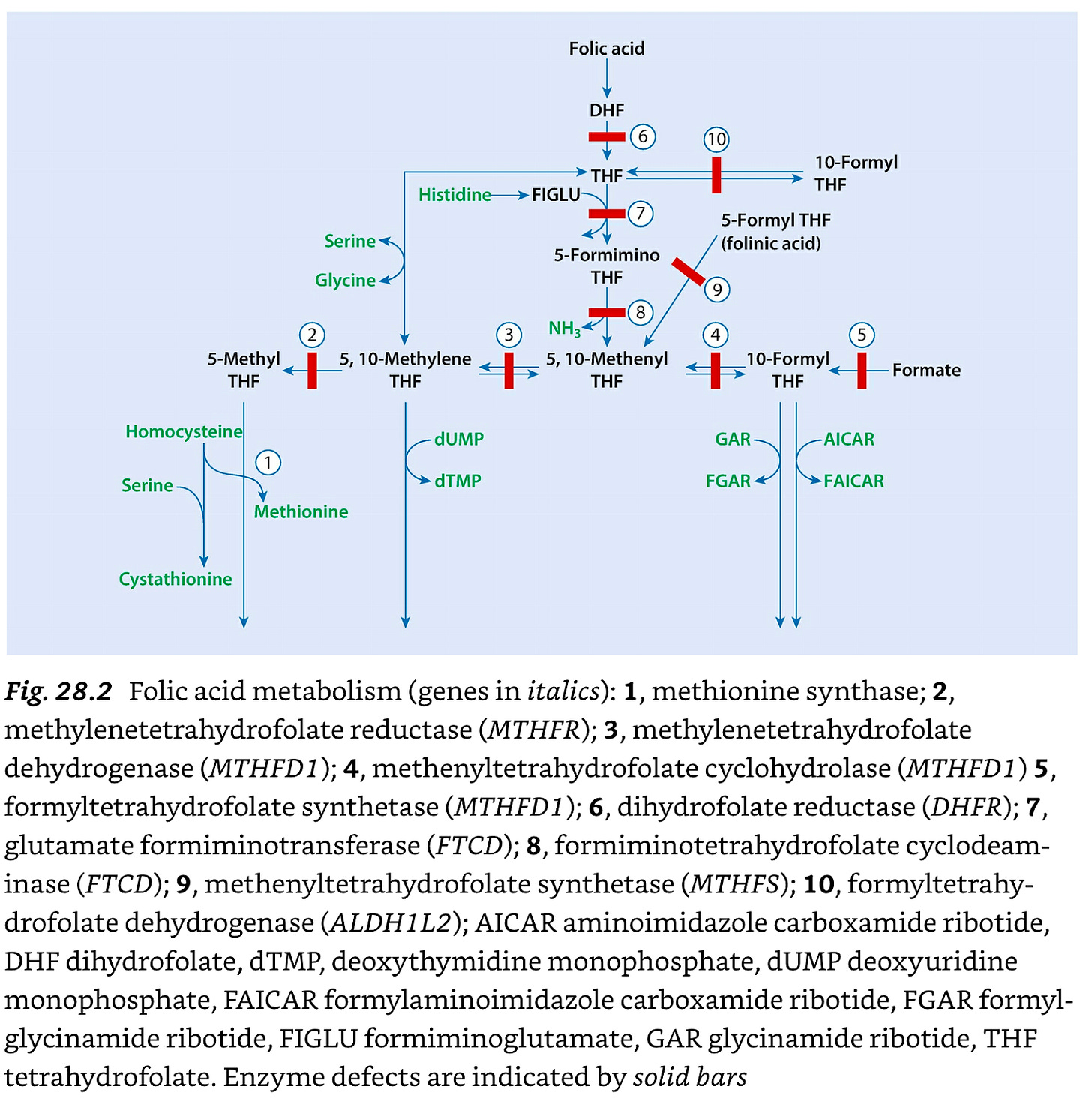
The Uses of Different Folates
The figure below is greatly simplified and is from chapter 28 of Saudubray, Inborn Metabolic Diseases: Diagnosis and Treatment, and shows the interconversion of different folates.
10-formyl-THF is used to produce FGAR and FAICAR, which are used for the synthesis of purines. This is mainly adenine, guanine, and all of their derivatives, such adenosine, ATP, GTP, the “A” and “G” bases of RNA and DNA, and the adenosine moieties included in the activated forms of B vitamins, such as NAD+ and NADPH (vitamin B3), FMN and FAD (vitamin B2), and CoA (vitamin B5).
5,10-methylene-THF is used for synthesizing dTMP, which is used for the “T” base of DNA.
5-methyl-THF is used for methylation.
5,10-methenyl-THF is, as far as we know, simply an intermediate between 10-formyl and 5,10-methylene-THF, while 5-formyl-THF, sold in supplements as “folinic acid” is a temporary release valve or storage form.
Not shown in the diagram, once 5,10-methylene-THF is used to synthesize dTMP, it generates DHF, which can be converted to THF by DHFR.
Not shown in the diagram, once 5-methyl-THF is used for methylation, or 10-formyl-THF is used for purine synthesis, they generate THF.
The THF from either of these processes can then undergo formylation to repeat the cycle.
Why THF Is Critical
THF is also important for glycine synthesis. During the conversion of serine to glycine, THF is converted to 5,10-methylene-THF.
Both of these processes are interconvertible. Thus, an accumulation of 5,10-methylene-THF at the expense of THF would encourage the conversion of glycine to serine.
THF is also necessary to detoxify formate. While formate is a normal part of metabolism, its safety is predicated on its incorporation into the THF molecule. If this does not happen, formate can build up and create a toxic burden.
THF is also necessary to clear the formiminoglutamate that occurs during the degradation of the amino acid histidine. Formiminoglutamate is not known to be toxic, but when it is elevated in urine it is a sign of deficient THF.
Do You Need to Consume THF?
If all aspects of your folate metabolism are working smoothly, it should only matter that you consume enough folate, not which form you consume.
However, if you have a block in THF production, this changes.
For example, suppose you have an impairment in MTHFR or in the downstream use of methyl groups. MTHFR is needed to convert 5,10-methylene-THF to 5-methyl-THF, and without doing that you cannot convert 5-methyl-THF back to THF.
This would cause the accumulation of 5-10-methylene-THF at the expense of THF, which would back up the conversion of serine to glycine and impair the detoxification of formate.
Or suppose you are improving your ATP status with creatine. Creatine will decrease the utilization of methyl groups, thereby increasing SAMe and shutting down MTHFR. Only the issue here is not that you can’t use MTHFR or methyl groups, it’s that you don’t need them.
If you aren’t using creatine, you need to read this article:
If you get any side effects from using creatine, that’s actually a sign you need it. Use my guide to handling creatine side effects here:
As described in that article, creatine has been shown to decrease glycine synthesis in humans, almost certainly through the mechanism I just described.
If you take a 5-methyl-THF (methylfolate) supplement, you can get around an impairment in MTHFR. But this won’t do anything to remove 5,10-methylene-THF. If it is used effectively in the methylation system, it could generate THF and thereby improve the ratio with 5,10-methylene-THF, and thereby improve glycine synthesis. But it still won’t help remove 5,10-methylene-THF and in fact it will probably make the accumulation worse, because by increasing the product of the MTHFR reaction you will decrease the flow through the MTHFR enzyme. The accumulation of 5,10-methylene-THF will negate any improvement in the level of THF.
If you have a block in the utilization of methyl groups, however, you could increase the amount of S-adenosyl-methionine (SAMe) available, and this will actually shut down the MTHFR enzyme. In proportion to the block of methyl group utilization, you will fail to increase THF. In proportion to the shutdown of MTHFR you will worsen the accumulation of 5,10-methylene-THF.
Adding in folinic acid might provide an advantage here in that it could generate THF in the synthesis of dTMP or purines, though the block in methylation would still impact it since one of the ways it would generate THF would be compromised.
But suppose you have a more general block, such as in ATP production. ATP is used in methylation, dTMP synthesis, and purine synthesis, so such an impairment would block all the paths toward THF regeneration.
In such a case, consuming THF would be a great way to keep glycine synthesis and formate clearance activated.
Synthetic folic acid could generate THF in these cases, but many people have too little DHFR activity to handle the load of folic acid that occurs in supplements and fortified foods. This is supported by the emergence of unmetabolized folic acid in the serum of Americans shortly after folic acid was added to bread in 1998, which has now become nearly ubiquitous. If the activity of DHFR is limited due to the genetics or epigenetics of the enzyme itself or due to a limit in the supply of NADPH, folic acid will fail to sufficiently raise THF. Further, there is still an outstanding question of whether unmetabolized folic acid in and of itself is harmful.
How to Know If You Need THF
On the Genova ION + 40 elevated formiminoglutamate and a high ratio of serine to glycine (the ratio isn’t calculated, but flagged high serine and flagged low glycine would clearly indicate the ratio is high, and you could also eyeball it for proportional position within the range, such as high-normal serine and low-normal glycine).
On the Vibrant America Micronutrient Panel, or on any test that includes both serum and red blood cell folate at the same time, low folate across the board suggests folate deficiency is the problem. Low serum folate and normal RBC folate suggests a backup in the methylation pathway is the problem.
This is because you could have virtually any nutritional deficiency impairing any of these pathways and you should always fix your weakest nutritional links before you try biohacking your way around a surface-level problem.
How to Get THF
Since there is no THF supplement on the market, the best way to obtain a targeted amount of THF is to juice romaine lettuce.
In general, getting a spread of liver, legumes, and greens prepared in various healthy ways — bonus for including some raw milk — is likely to produce a complex exposure to the different folate metabolites, which is a more robust way to obtain folate than using supplements, because the diversity of folate forms can protect against unidentified backups in the metabolic pathways.
If you know you need THF, juiced turnip greens, beet greens, broccoli, and romaine are reliable ways to obtain it, with romaine being the safest of these to push the dose on.
The practical details are covered in Why You Need to Start Juicing Tonight: The Sleep-Anxiety Connection Nobody's Talking About.










Thank you for giving me an unexpected anxiety attack. This brings back my college days. Not so much the chemistry, but my fraternity days. Geez!
Thanks a lot for this. I always had the feeling that I dont quite get folate and this made it much clearer!