The Central Role of Thyroid Hormone in Governing LDL Receptor Activity and the Risk of Heart Disease
In “Genes, LDL-Cholesterol Levels, and the Central Role of LDL Receptor Activity in Heart Disease,” as well as my most recent presentations at Wise Traditions and AHS, I described the overwhelming genetic evidence for the theory that LDL receptor activity centrally governs the risk of heart disease and the large amount of other evidence from human and animal experiments that offers further support for this theory.
If we look at the factors that govern how many LDL recpetors our cells make, we immediately begin to suspect that thyroid hormone may play a central role in providing immunity to heart disease.
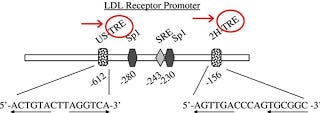
Here is a diagram of the promoter region for the gene that codes the LDL receptor (1):
Figure taken from Lopez D, Abisambra Scarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771(9):1216-25.
If you would like to enlarge the picture, you can do so by clicking on it.
This is a diagram of the promoter region of the rat gene, but the human gene is structured similarly (2). The arrows point to two parts of the promoter marked “TRE,” circled in red. “TRE” stands for “thyroid response element” and is the name we give to sections of DNA where thyroid hormone and its receptor bind.
In between the two thyroid response elements, we also see a section marked “SRE.” This is a “sterol regulatory element.” It's role is to respond to the level of free cholesterol in the cell.
We can thus see that the cell regulates its expression of the LDL receptor for two reasons. It cares about its own needs for cholesterol, and it cares about the body's need for bile acids, sex hormones, and other things made from cholesterol.
If the cell doesn't have enough of its own cholesterol, it communicates this through the sterol regulatory element (SRE). Once the cell activates this section of DNA, its gene expression machinery will descend upon the gene and begin making LDL receptors. These will bring cholesterol-rich LDL particles in from the blood and restore the cell's level of cholesterol.
Thyroid hormone, by contrast, is an endocrine hormone. It's job is long-range communication. Through the brain and some closely related glands, the well-fed body determines that it is in a state of abundance and communicates this to cells using thyroid hormone. Even if a liver cell or adrenal cell has plenty of cholesterol, thyroid hormone comes with trumpets and fanfare announcing that it's time to collect even more cholesterol so the cell can ramp up its production of bile acids, sex hormones and other goodies. It binds to its receptor, activates the thyroid response element (TRE), and once again the cell's gene expression machinery descends upon the gene and begins making LDL receptors.
Many cholesterol-lowering drugs such as cholestyramine and the statins work their black magic by lowering the level of free cholesterol in the cell. The cell reacts to this deficiency by increasing its production of the LDL receptor through the SRE. More LDL receptors means more LDL-cholesterol will be cleared from the blood in a shorter amount of time, and this will help prevent the oxidation of those LDL particles, which will in turn reduce the burden of atherosclerosis, all things being equal. But these drugs do nothing to ramp up the level of cholesterol-made goodies to promote strength, proper digestion, virility and fertility. It is the vocation of thyroid hormone, by contrast, to do both.
Cholestyramine and statins have some nasty side effects, some of which can actually promote heart disease. Since I've already sold out to the USDA (I hope my overlords don't find this), I don't want anyone to think I'm actually pushing these drugs!
In any case, we could use our map of the LDL receptor promoter region to predict that correcting thyroid hormone deficiency would protect against heart disease. In fact, decades before we knew anything about the LDL receptor, a physician and medical professor by the name of William Kountz published a clinical trial showing that correcting thyroid hormone deficiency nearly abolished the risk of heart disease (3).
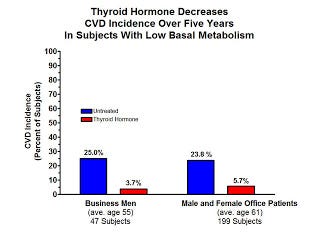
Kountz selected three groups of people with a low basal metabolic rate. The first group consisted of just under 50 business men with an average age of 55; the second group consisted of just under 200 office patients, some men and some women, with an average age of 61; the third group consisted of poor elderly people with an average age of 67, all of whom were confined to an infirmary and had advanced heart disease.
Kountz gave all his patients B vitamins, but he gave some of them thyroid extract and let others serve as controls. He advised both the thyroid-treated patients and the controls to quit smoking and to be “moderate” in their consumption of food and alcohol. He gave the treated patients just enough thyroid extract to normalize each individual's basal metabolic rate.
Thyroid treatment proved ineffective in the poor elderly infirmary patients with advanced vascular degeneration, but here are the results in the other two groups with less advanced disease:
Thyroid extract reduced the incidence of heart attacks by four-fold in the older office patients and by seven-fold in the younger business men. Stated another way, it reduced the incidence of heart attacks by 76% among the older office patients and by 85% in the younger business men. This effect is much more powerful than that seen with any drugs that are currently on the market and suggests that correcting thyroid deficiency may be able to nearly abolish the risk of heart disease if done so early enough.
Kountz published his results in 1951, over two decades before Brown and Goldstein discovered the LDL recpetor pathway. Thus, Kountz had no way of knowing the role of thyroid hormone in regulating the expression of this receptor, and no way of knowing the critical role of this receptor in governing the risk of heart disease. He suggested that thyroid hormone was especially protective against degeneration of the media, the layer of the blood vessel that lies behind the intima, where atherosclerosis takes place.
Thyroid hormone may indeed protect the media from degeneration, but it is difficult not to view his experiments in light of the role we now know thyroid hormone plays in regulating production of the LDL receptor, thus protecting LDL particles from oxidation and thereby preventing the development of intimal atherosclerosis.
Nikolai Anitschkov had identified thyroid hormone as a protective factor decades earlier based on his experience with the cholesterol-fed rabbit model, and this was one of several protective factors he listed in his combination theory of atherosclerosis. I discussed this theory in my post “The Origin of the Lipid Hypothesis — And Proposal of a New Term.” Anitschkov was truly a man ahead of his time.
It would appear from Kountz's results that thyroid hormone is a central governor of heart disease risk in patients with a low basal metabolic rate. It also seems that the basal metabolic rate is a good test for identifying people whose risk of heart disease can be nearly abolished merely by boosting thyroid status. I would then say that in a large sector of the population, the degeneration of lipids in the blood is caused by a form of miscommunication, where the body is in a state of abundance, but it is unable to communicate that message to its cells.



Reminds me of Dr. Broda Barnes' findings on thyroid hormone and heart disease - https://youtu.be/YFEGcOo-QpQ?t=518