How Serotonin Helps You Breathe
Installment four in our series on understanding the truth about SSRIs.
We cannot understand what SSRIs are really doing without understanding how serotonin is impacting mitochondrial energy metabolism.
Yet, we also cannot truly understand that either unless we first situate it in the context of serotonin serving as the traffic cop of our lungs.
Therefore, the fourth installment of our series covers this traffic cop role.
Every thing we do — whether sleeping, eating, exercising, listening, imagining, or creating — requires oxygen from the air we breathe. That oxygen travels to the heart of complex IV in the mitochondrial respiratory chain and facilitates our production of cellular energy in the form of ATP. That cellular energy fuels everything.
When we take in a breath, some parts of our lungs are more oxygenated than others. Which parts are most oxygenated changes over the course of the breath as the oxygen is removed into our blood.
If our blood perfuses a poorly oxygenated area of our lungs expecting to find oxygen, not only will it be disappointed but we can develop a form of “ventilation/perfusion mismatch” (“V/Q mismatch”), which can cause hypoxemia (low blood oxygen), shortness of breath, fatigue, dizziness, and confusion.
While typical discussions of V/Q mismatch focus on not having the right ratio of ventilation (breathing) to perfusion (blood flow), here we are discussing a form of V/Q mismatch due to spacial distribution of the blood: the blood is perfusing into the areas that are poorly ventilated (oxygenated) instead of selectively perfusing the areas that are well ventilated.
Serotonin acts as the traffic cop: it shuts down the flow of blood into poorly oxygenated areas and redirects it to flow into the well oxygenated areas.
This allows you to avoid wasting blood flow where it won’t have high oxygen payoff, thereby keeping high return on investment for each pump of blood you engage in and each breath you take.
This also has a dark side: if you are subject to chronic hypoxia, serotonin will facilitate the remodeling of the vasculature of your lungs in a way that promotes pulmonary hypertension, which can often feel like hypoxia or V/Q mismatch except it often is accompanied by chest pain, and it can become chronic or quasi-permanent.
In this article, we explore the science of how serotonin helps make every breath worth taking.
Serotonin and Its Receptors
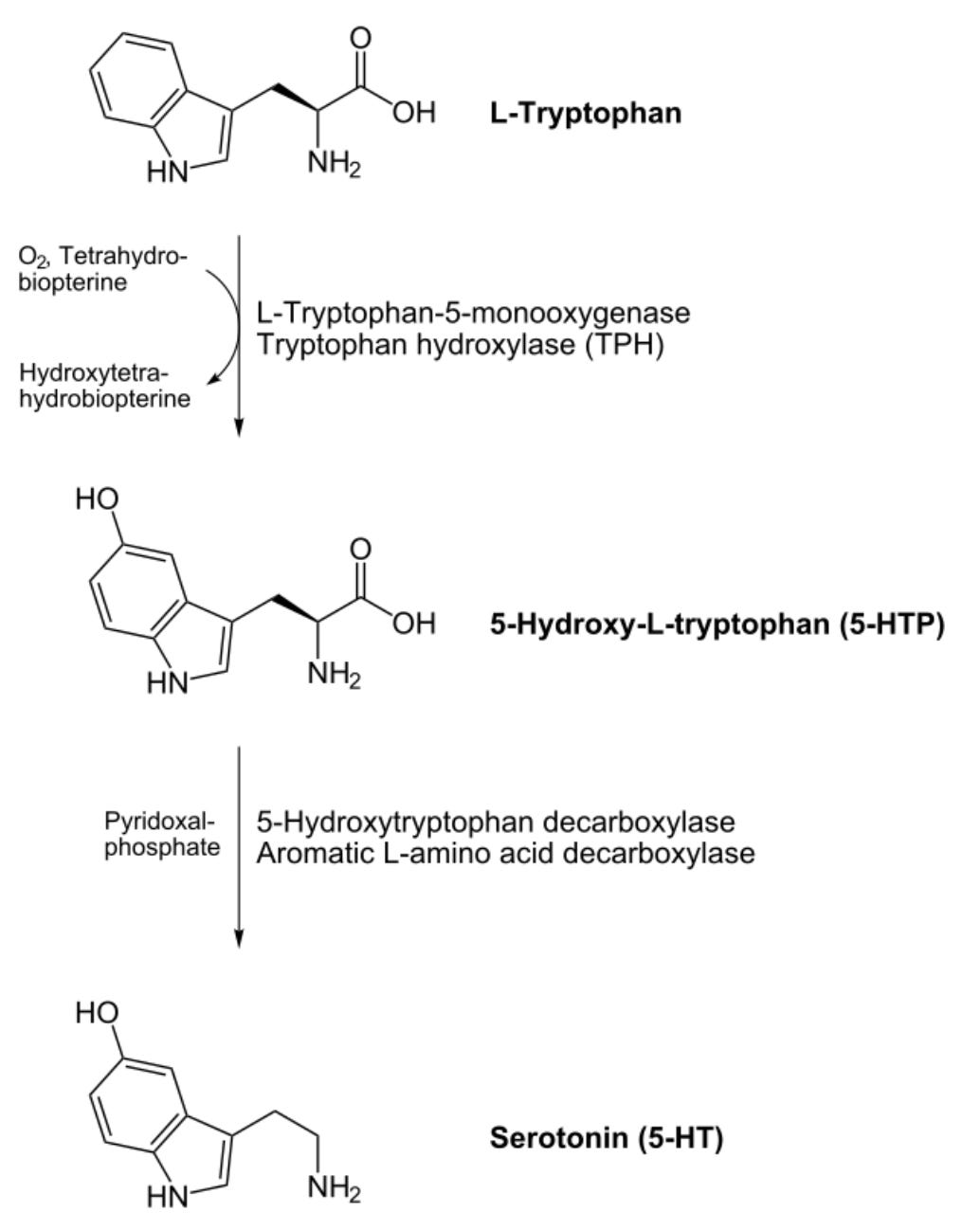
Serotonin is derived from the amino acid tryptophan in two steps involving adding a hydroxyl group (OH) to its ring structure and removing the carboxyl group (COOH) that makes it an acid. Because the carboxyl group is removed it is no longer an amino acid but rather an amine, and its alternative name better reflects its structure and derivation, 5-hydroxytryptamine (5-HT).
Serotonin acts in the nervous system, gut, lungs, blood, heart, adrenals, bladder, and immune system, and many other tissues by binding to a receptor on the cell surface that then elicits a response in the target cell.
While pharmacology and psychiatry primarily regard the serotonin transporter as a means to remove serotonin from the neuronal synapse to lessen its activity, cells that respond to serotonin also use the serotonin transporter to take serotonin inside the cell where it can bind to serotonin receptors on a number of different organelles, including mitochondria.
As we covered in the previous installment, serotonin transporters are NOT found mostly, primarily, or even disproportionately in the brain. Outside the brain they are not found mostly, primarily, or even disproportionately in neurons. Serotonin and its transporter are primarily non-brain, non-neuronal molecules.
There are at least 15 different serotonin receptors divided into seven different families, most of which have two or more subtypes. These include 5-HT1 receptors, subtyped A through F; 5-HT2 receptors, subtyped A through C; 5-HT3A and B receptors; 5-HT5 A and B receptors; and then three families that each have only one lone receptor per family: 5-HT4, 6, and 7.
This diversity of receptors allows downstream responses to serotonin to be regulated differently by serotonin levels as well as by other signals, allowing serotonin to perform many different functions in different contexts.
The diversity can be further enhanced by different serotonin receptors binding to one another in complexes, by them forming complexes with other unrelated receptors, and possibly by two of the same receptor types binding together in a complex.
5-HT3 receptors allow sodium and potassium ions to flow through when activated by serotonin, which creates a change in the voltage across a membrane that induces downstream signaling.
All other known 5-HT receptors are coupled to intracellular signaling cascades mediated by the activation of G proteins, which are very common orchestrators of such cascades throughout metabolism.
5-HT1A and B receptors are notable for their occurrence on the axons, bodies, and dendrites of serotonin-releasing neurons where they form “autoreceptors” that regulate serotonin release and reuptake partly as a form of negative feedback against excessive serotonin signaling. These same receptors, however, can also be found on post-synaptic dendrites where they facilitate serotonin signaling in the downstream neuron, playing roles in regulating aggression, anxiety, addiction, appetite, memory, learning, mood, sleep, and the perception of pain and temperature.
5-HT3 and 5-HT4 receptors are especially important in the gastrointestinal tract, where they stimulate gastrointestinal motility but in excess can cause diarrhea and vomiting. However, they too are found in the central nervous system where they play roles in cognitive function, emotions, appetite, pain, and the gag reflex.
The functional roles of the diversity of receptors are overlapping and extremely complex, making them difficult to analyze and a perpetual fertile ground for new discoveries.
Serotonin’s Role in the Whole-Body Response to Hypoxia
The acute whole-body response to hypoxia is mediated by a homeostatic oxygen-sensing system composed of neuroepithelial bodies in the lungs, glomus cells of the carotid bodies of the carotid artery, smooth muscle cells of the pulmonary arterioles and capillaries; and the systemic arteries.
Within fractions of a second, the opening and closing of ion channels has acute effects on factors such as the dilation or constriction of blood vessels and the rate of breathing; over the course of minutes and hours, changes to gene expression take place, with varying responses over the course of less than eight hours, more than eight hours, or long-term effects of chronic constant hypoxia or chronic intermittent hypoxia.
When hypoxia becomes severe enough, all cells of all tissues will participate in the response to their own level of hypoxia, and some cells are chronically hypoxic due to low blood supply, such as cells of the lens, cornea, and testes.
That serotonin is involved in the hypoxia response is suggested on the surface by the fact that serotonin syndrome – a dangerous constellation of symptoms caused by excessive serotonin exposure – is usually accompanied by an increase in the breathing rate.
The clearest role of serotonin in the hypoxia response is in the signaling of the neuroepithelial bodies of the lungs, which are innervated cell clusters with built-in oxygen sensors. The fact that these are most prominent around the time of birth and decline in density steeply thereafter has led to the hypothesis that they are primarily important in the neonatal period. However, while they might be especially important during that period, they do not decline to zero density, and animal experiments suggest they are important in adults. For example, adult mice with genetic deletions in their hypoxia response display overgrowth of these bodies. Adult mice with genetic deletion or pharmacological inhibition of the 5-HT2B receptor, moreover, fail to engage in the tissue remodeling that otherwise occurs in response to chronic hypoxia, which is the basis for the pulmonary hypertension that occurs with such remodeling. These findings lean in the direction of these bodies retaining importance in adults.
The oxygen sensing in these bodies depends on hydrogen peroxide produced from oxygen primarily by the enzyme NADPH oxidase in the cytosol and by the respiratory chain in the mitochondria. NADPH oxidase uses riboflavin (vitamin B2) in its activated form of flavin adenine dinucleotide (FAD) and the niacin (vitamin B3)-derived reduced nicotinamide adenine dinucleotide phosphate (NADPH) to convert oxygen to the free radical superoxide, which is converted to hydrogen peroxide by the zinc- and copper-dependent cytosolic superoxide dismutase. The mitochondria of these cells also make superoxide in proportion to oxygen flux that is converted to hydrogen peroxide by the manganese-dependent mitochondrial superoxide dismutase.
This hydrogen peroxide oxidizes a cysteine residue in a potassium channel, which keeps it open. Hypoxia leads to an acute drop in hydrogen peroxide that closes the channel, triggering a change in the voltage across the membrane, stimulating a calcium influx that initiates release of serotonin.
Part of this serotonin travels up the vagus nerve to the brainstem where it can interact with brainstem centers that regulate breathing. Mice lacking the serotonin transporter, and thus having elevated extracellular serotonin, have a higher breathing rate both at normal oxygen levels and during hypoxic exposure. While it is not statistically significant, they seem to have a slightly exaggerated increase in the breathing rate in response to hypoxia as well.
The increase of the breathing rate during exposure to normal oxygen levels could reflect participation of extracellular serotonin in the basal tone of the hypoxia response, which is never dialed down to zero.
Collectively, the findings above show that serotonin mediates a part of the response to both acute hypoxia by increasing the breathing rate and chronic hypoxia by mediating tissue remodeling.
The impact of serotonin in hypoxia is not limited to that secreted by the neuroepithelial bodies into the vagus nerve:
Blood vessels respond to serotonin, as clearly demonstrated by the impact of 5-HT2B receptor inhibition on hypoxia-induced vascular remodeling.
Carotid bodies contain serotonin and possess serotonin receptors.
The raphe magnus, a part of the brainstem that serves as a major source of serotonin within the central nervous system, has anatomical connections to the parts of the brainstem that control the breathing rate, and hypoxia increases their production of serotonin. In rats, injecting an agonist of the 5HT1A receptor directly into this structure lowers serotonin production; this has no effect on the breathing rate during exposure to normal air, but it blunts the increase in the breathing rate in response to hypoxia.
Hypoxia increases platelet activation, and when platelets activate they release serotonin. Therefore, it is likely that platelets release serotonin into the general circulation during hypoxia.
Serotonin that is in the blood outside of the platelets is called “circulating free serotonin.” In preterm infants, those with circulating free serotonin had half as many hypoxemia events as those without any detectable free serotonin, consistent with circulating free serotonin protecting against hypoxia.
Synthesizing all this, the secretion of serotonin in response to hypoxia starts as soon as a neuroepithelial body in the lungs senses a hypoxic breath. This is then increased further as hypoxia spreads to the carotid bodies of the carotid artery and then to the raphe magnus of the brainstem. As the systemic circulation becomes hypoxic, platelets release serotonin into it that can be taken up by any tissue.
Serotonin promotes vasoconstriction of the pulmonary arteries during hypoxia by acting directly on smooth muscle cells. All other things being equal, constricting a blood vessel raises blood pressure by increasing the tension where the blood pushes against the blood vessel wall. Thus, this is a component of the pulmonary hypertension that can develop during hypoxia.
However, hypoxic pulmonary vasoconstriction is absolutely essential in day-to-day breathing because this is mechanism by which blood perfusion is matched to well-oxygenated lung segments.
As a specific segment becomes relatively hypoxic compared to other segments, serotonin locally constricts the blood vessel, conserving blood to flow into the better-oxygenated segments.
Thus, serotonin plays a role as traffic cop, helping direct the blood away from poorly oxygenated lung segments and conserving blood flow for where the best oxygen payloads are found.
Now that we properly understand how entwined serotonin is in the behavior of our lungs during hypoxia and in the whole-body physiological response to hypoxia, we can better discuss the science of how serotonin impacts the mitochondria.
That is the topic of the next installment. Read it here:
Your Mitochondria and the Two Faces of Serotonin
In our series on how to understand the truth about serotonin, SSRIs, SSRI side effects, and the sometimes devastating effects of SSRI withdrawal, we have so far established that serotonin is a primarily non-brain non-neuronal signaling chemical that is fundamentally involved in the hypoxia response, and is also the substance from which we make melatonin…





Can you elaborate more on the potassium sodium pump? How do ssris effect the electrical part of the heart?
Thanks, Chris, for this.
I did not know you had a Substack and Serendipity led me here.
Will be following your work with gratitude.