If you’re having issues with playing the video above, play this instead:
The first lesson in the course is free to everyone.
The first MWM Energy Metabolism lesson answers the question, why do we have to eat such an enormous amount of food?
The answer is to comply with the second law of thermodynamics. If you have a chemistry background, you should recognize this as a light review of the thermodynamics unit from a general chemistry class, with its most essential concepts teased out and packed into a half-hour lesson. If you don't, you can use this as a basic foundation for understanding the biochemistry to follow.
The lesson relates the 2nd law to food coloring dispersing in water, how a hydropower plant operates, ATP production, and why we need to eat our body weight in food more than once a month. In the process, we have a little fun.
Listen to the Audio
Listen to the audio here.
Transcript, Slides, References, Further Reading
These materials are free to everyone for lesson 1. Members of the Masterpass have access to transcripts, slides, references, and further reading for the entire course. To learn more about the Masterpass, click here.
Read the transcript and other materials below, or download them here.
Hi. I’m Dr. Chris Masterjohn of chrismasterjohnphd.com. And you are watching Masterclass with Masterjohn.
We are today going through the first in a series of lessons on the system of energy metabolism. Now energy metabolism is so fundamental to biology that this system underlies and supports every other system in the body.
So no matter what you value about your health, whether it’s protection from degenerative diseases as we age, or it’s supporting athletic performance, mental performance, whatever it may be.
Whatever we value about our health all depends on our ability to extract energy from food and use it effectively. So without further ado, let’s just jump right into the lesson.
Video Time: 0 min 55 sec
I’d like to start this discussion by posing a question. Why do we have to eat such an enormous amount of food? Shown on the screen is this little fella who weighs 150 pounds. And he eats 2,000 pounds of food per year.
Related: NPR: The Average American Ate (Literally) A Ton This Year
Now I know what you’re thinking: “That dude is a slob.” But hey wait a second, you do it, I do it, we all eat our body weight in food more than once a month. That seems crazy.
Why can’t we just eat our body weight in food once and be done with it? Well I know what you’re thinking: “I gotta go to work, I gotta go to school, I gotta go see my friends, I gotta go to the gym.”
Everything that we do involves physical activities that require new inputs of energy. But that doesn’t explain it. If we get lazy and do nothing, we still get hungry. If you imagine lying in bed for three days straight, you’re not going to go on a water fast, you’re going to get up and go to the refrigerator on multiple occasions.
So it’s not just the things that we do. Now okay, okay, I know what you’re saying: “But my heart is beating even when I’m sleeping and my lungs are always breathing.” Those things are important and they do require a lot of energy.
However, there’s more to it than that, and you can see this easily by thinking about how much heat we evolve into our environment. We don’t usually notice it, but you would certainly notice it if you stopped yourself from evolving heat into the environment. Just put on a sweater, and then a hoodie, and then an overcoat, and then a blanket, and a wool hat and wool socks. You’ll see what I mean pretty fast.
You will get hot in no time, and that’s because this massive quantity of heat that you’re always just leaving to go out into the atmosphere is now starting to accumulate. So there’s gotta be more to it than this.
In order to really understand what’s going on here, we need to think about thermodynamics. The first law of thermodynamics states that the energy in the universe always remains constant. It can change forms, but it can’t be created or destroyed. That doesn’t really seem to explain anything though.
Because if energy is fundamentally conserved, why can’t we use the energy from one heartbeat to fuel the next; and then use it again to fuel the next one, and the next one, and the next one?
Are we just remarkably inefficient because we’re not recycling the energy properly? To really understand this, what we have to talk about is the second law of thermodynamics.
Video Time: 03 min 44 sec
Related: Catalysis and the Use of Energy by Cells
For a much more detailed treatment, see chapter 20 in Silberberg, Chemistry: The Molecular Nature of Matter and Change
The second law of thermodynamics holds that the entropy of the universe, which we can think of as the disorder of the universe, is always increasing. And in any given system, the system will tend towards ever greater disorder unless energy is invested into that system to create, maintain and repair order within it.
In order to grapple with this on an intuitive scale, we can think of a messy room. Shown on the left is a messy room; on the right is a clean room. And we can look at that and say, you know what, I’d have to invest a lot of energy to maintain my room always looking as clean as the one on the right. If I get lazy and I’m not investing my energy into cleaning my room, it’s going to start to look like the one on the left.
We can also imagine that if there were a series of spontaneous accidents, it’s going to tend to produce something like the room on the left instead of the one on the right. If a huge gust of wind came through that room and blew things around, things are going to look like the room on the left.
One of the defining characteristics about the room on the left is that the items in the room are randomly distributed. The probability that a book is going to be on the table is relatively similar to the probability that it will be on the floor or on the chair versus in the drawers. By contrast, the room on the right has all of its items distributed in a highly non-random fashion. The books are in the drawers, and they’re not found anywhere else.
Video Time: 05 min 23 sec
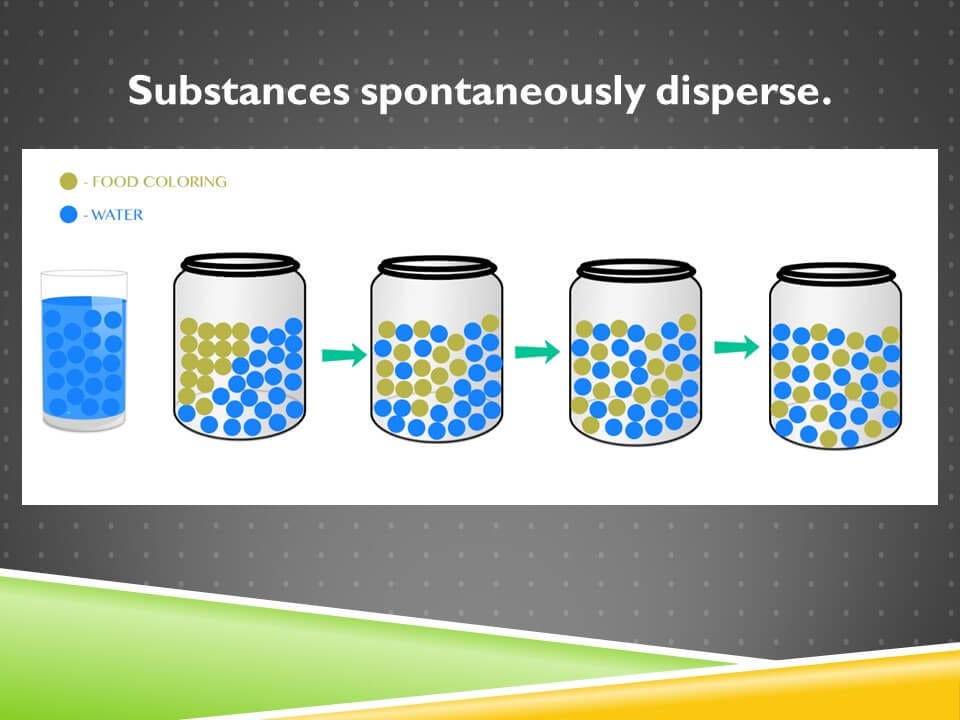
Let’s look at this from a more microscopic level. Shown on the screen is a glass of water and food coloring is dropped into the water. It starts off highly concentrated in the left corner, but then it disperses over time until it’s randomly distributed.
If you look at the jar on the right, it may not immediately look disordered to you, but if we use the definition I offered before, that the items within a space are randomly distributed, it absolutely qualifies.
On the left, all the food coloring is in the upper left corner and none of it is in the lower right corner. But in the jar on the right, if you take any two locations, compare the upper left to the lower-right, compare the middle to the side, the likelihood of finding a food coloring molecule there is approximately equal.
From a thermodynamic sense then, the system on the left is high in energy and it’s high in order. The system on the right is low in energy and low in order. It would require organized effort and energy to produce the system on the left, and that’s not going to happen spontaneously. By contrast, systems spontaneously become less ordered and lower in energy over time.
Now just as we have to be careful with the word “order” when we’re talking about it in this thermodynamic sense, we have to be careful with the word “spontaneous.” In thermodynamics we can define spontaneous as that which happens in the absence of an input of energy.
Now there’s a couple things going on here. One is a mere matter of probability. In any given substance, the molecules within it have some energy that’s causing the molecules to move around.
If that energy doesn’t have any direction to it, then they’re as likely to go up as down, as left or as right. And so anything given space to move around in and given that level of directionless movement will tend to randomly distribute itself over time.
In this jar, that’s accelerated because the food coloring has reached the boundary of the jar on the left side. If the food coloring moves to the left, it has nowhere to go, and it’s just going to bounce back and go to the right. So all the more rapidly will the food coloring tend to occupy the space that it hasn’t occupied yet over time.
But there’s something more to it than that as well. Within nature there are many attractive forces. Explaining them is well beyond the scope of this lesson, but there’s attractive forces between things that are water-soluble and water. In this case the food coloring is water-soluble and so it’s attracted to the water.
And again we can define it as follows: things will spontaneously move toward and interact with the things that they’re attracted to and when they do so they achieve a less ordered and lower energy state. These food coloring molecules in the upper left, most of them aren’t interacting with water, and they can’t until they spread themselves out.
On the right, each food coloring molecule is able to maximize its interaction with water because they’re spread out. Because we can have one, two, three, four water molecules interacting with a single food coloring molecule. So from a thermodynamic sense things will tend to follow the course of their attraction spontaneously and it will take energy to prevent them from maximizing their interactions with the things that they’re attracted to.
Now we are nothing like the jar on the right. If you look at me right now, you can see that my eyeballs are not equally likely to be on the top as the bottom of my head, my arms are not equally likely to be coming out of my ears as my torso. If we look at each other, we can easily see that we are highly ordered beings.
Once we start looking at what’s going on inside the cell, then there’s a stunning level of organization and complexity that’s absolutely necessary for life. But there’s a little problem. If we are investing energy in creating order within ourselves, then that is okay by the second law of thermodynamics.
But the problem is that the second law of thermodynamics says the total disorder in the universe is always increasing. If we’re going to add to the order in the universe by creating order within ourselves, we need to compensate for that by creating a greater amount of disorder in our surroundings, in order to comply with the second law of thermodynamics.
Video Time: 10 min 34 sec
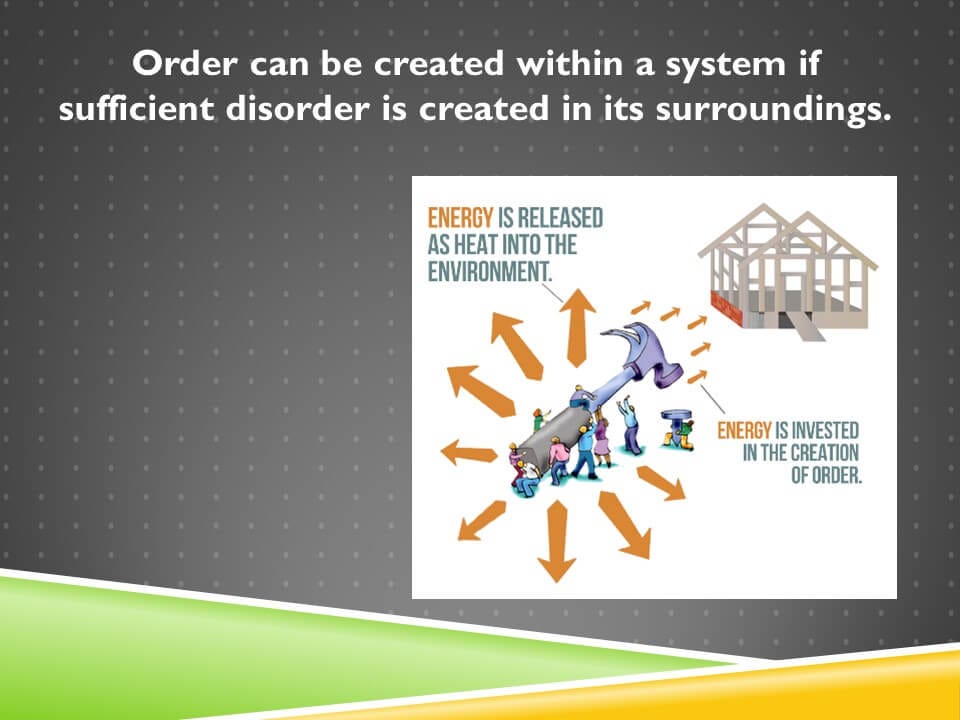
The cartoon on the screen demonstrates this principle. These people are building a house. That house is orderly. In order to build it, they need to invest energy in the house as symbolized by these small orange arrows. But in order to compensate for that order they need to release a greater amount of disorder into their environment, and that’s accomplished by releasing heat into the environment.
And the fact that these arrows symbolizing the heat are much bigger than the arrows symbolizing the energy invested in the house reflects the fact that we need to overcompensate for the order so that the net change is always in favor of more disorder in the universe.
Video Time: 11 min 24 sec
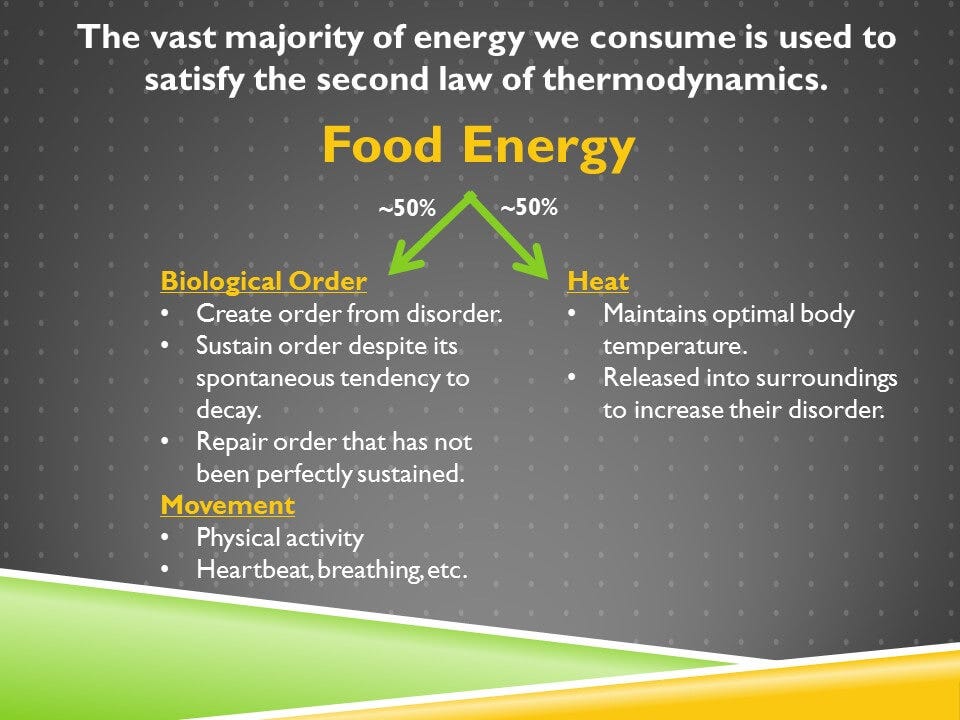
So let’s relate these principles to the energy that we derive from food. We take about 50% of that energy and harness it to do useful things in the body. A very large bulk of that 50%, is all investing in order. It’s creating order from disorder. That requires energy because of the second law.
Once that order is created, we need to invest more energy in sustaining it. Because without an energy input, it tends towards decay. That’s because of the second law. Some of that order will escape our maintenance and become damaged and we’ll need to repair it, again because of the second law.
Now part of the energy that we harness is also for our physical activity and the internal movements, like our heartbeat and our breathing.
But half of the energy that we don’t harness is just released as heat without doing anything useful with it. And yeah we need that heat to maintain our optimal body temperature, but we could be better insulated. We’re not. But we need to release that energy into our surroundings because all of the energy that we invest in creating and sustaining order within ourselves, must be compensated for with a greater amount of disorder in our environment.
That disorder is achieved by releasing heat, the heat supplies the energy for random directionless motions of the molecules in the atmosphere.
And, therefore, this 50-50 utilization of energy is not an inefficiency. In fact, if you compare this to a car, a car is harnessing about 20% of the energy from the burning of the gasoline, and that car doesn’t have to create order, that car doesn’t repair itself, that car just needs to get from point A to point B, and it’s only harnessing 20% of the energy.
We need to create all this order in addition to our movements and that creates a responsibility on us to comply with the second law by evolving more heat into the atmosphere to compensate for that order. That’s stunning efficiency.
Related: Section 1 of “How Cells Obtain Energy From Food” in Molecular Biology of the Cell compares and contrasts our metabolism with that of an automobile.
Video Time: 14 min 00 sec
If we have to invest energy into the creation of order, then that implies that the system that we’ve made orderly now is high in energy, and that that order and that energy can be released to do work at a later time point.
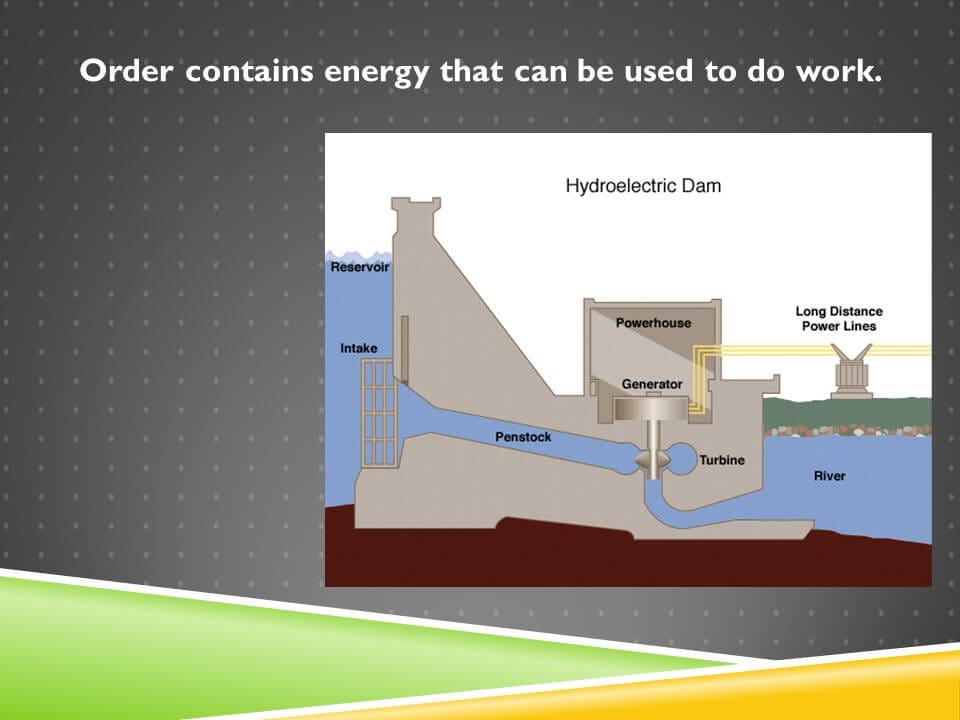
The cartoon diagram of a hydroelectric dam on the screen illustrates this point. Humans invest energy in creating a dam. That dam is meant to hold water in a high position.
One of the attractive forces of nature is gravity. The water wants to go from a high position to a low position. And when it does so, it will become lower in energy and order and will release that energy.
The purpose of the dam is to control that flow of energy so it can be harnessed to do work. When the intake is let open and the water flows through the penstock, the potential energy of the water by virtue of its high position, is now released as the kinetic energy of the movement of the water and it turns a turbine. And that turbine turns with the kinetic energy that’s been translated into electrical energy. And then is stored for use at a later time point. Again as the potential energy within the electric power system that powers society.
Video Time: 15 min 33 sec
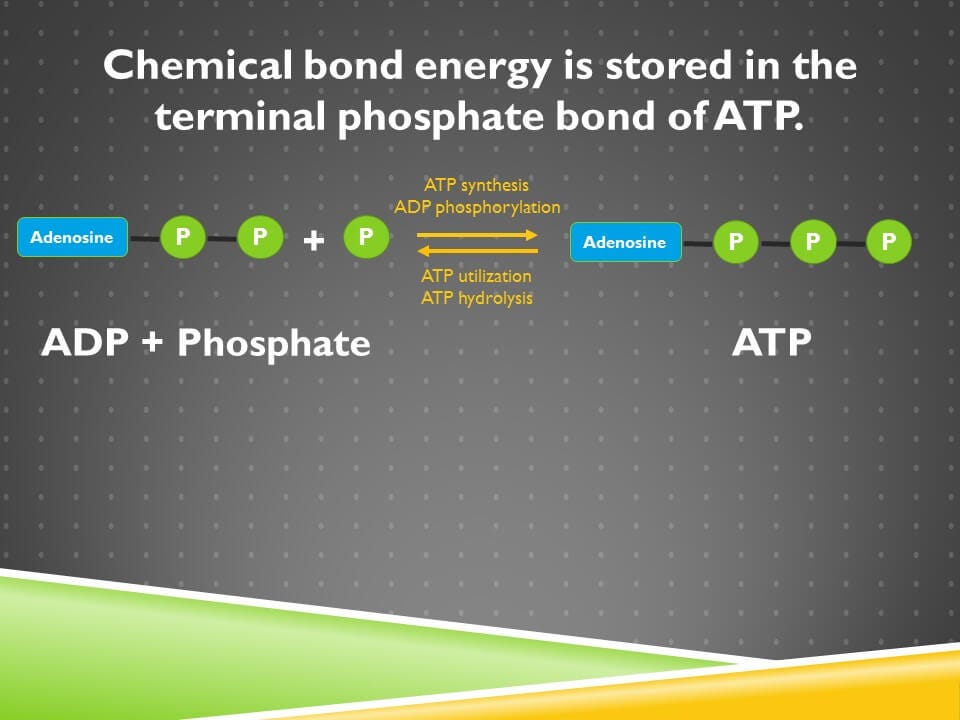
Electricity is a major energy currency of our society. The chemical bond energy of ATP is the major energy currency of our cells. ATP synthesis and utilization is shown on the screen in a very abbreviated format.
ADP is adenosine diphosphate; it’s adenosine with two phosphate groups. If we add a third phosphate group to it, that’s called ADP phosphorylation and it produces adenosine triphosphate, so it’s also called ATP synthesis. We can use the energy stored in that terminal phosphate bond by hydrolyzing it, and that’ll make us go leftward back to ADP and phosphate. That’s called ATP utilization or ATP hydrolysis.
Now, chemical bonds form because of attractive forces of nature that are beyond the scope of this lesson, but what we can consider here is that these are all the same atoms on the left and on the right and they are two different configurations of those atoms. There’s a natural balance that these atoms will want to achieve in the relative possibilities of their configurations between these two states. Just like there’s a relative distribution that the food coloring molecules would want to achieve throughout the glass of water that they could occupy.
Related: The Concentration of Reactants Influences ΔG
Chapter 20 in Silberberg, Chemistry: The Molecular Nature of Matter and Change provides a detailed foundation for understanding equilibrium.
However, for reasons that are well beyond the scope of this lesson, the relative balance of ADP and phosphate and ATP isn’t going to be an exactly random distribution. But it’s going to be some kind of equilibrium, where if we just left ADP and ATP to their own devices and we didn’t put in any energy into the system, then there would be some rate of ATP synthesis and some rate of ATP hydrolysis that would equilibrate to an equilibrium value where they’re at their lowest energy state.
So the purpose of ATP synthesis, then, isn’t to get some intrinsic value in that third phosphate bond. It’s to make that third phosphate bond have a lot of value by synthesizing ATP at such an enormous rate that we maintain the relative concentrations of ATP and ADP so vastly far out of equilibrium, that there’s enormous desire, enormous amount of energy that could be released from the ATP being hydrolyzed.
And in this sense, we can kind of think of it like a bow and arrow. When you start to pull on the bow, there’s a little bit of potential energy that’s stored in the string. But where you’re really going to get the big bang for the buck, where you’re really going to get a strong punch out of that, is as you expand it further and further and further until there’s a lot of tension there. Because that bow wants to go back to the equilibrium value of the resting state, so you pulling it away from that equilibrium is exactly what gives it so much power that it can shoot the arrow to your desired target.
Video Time: 19 min 26 sec
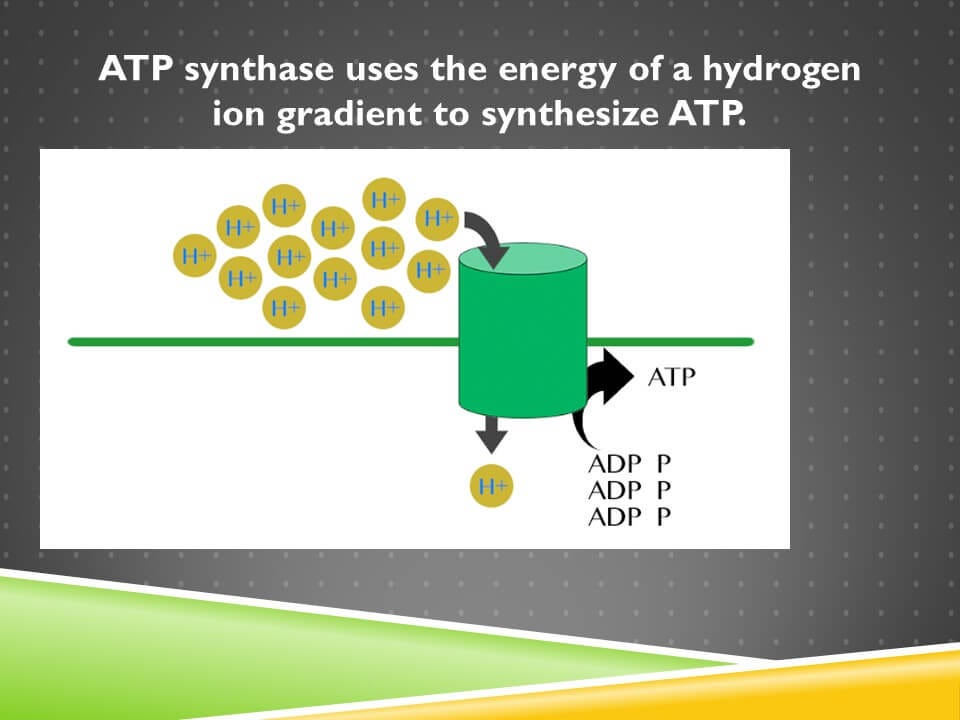
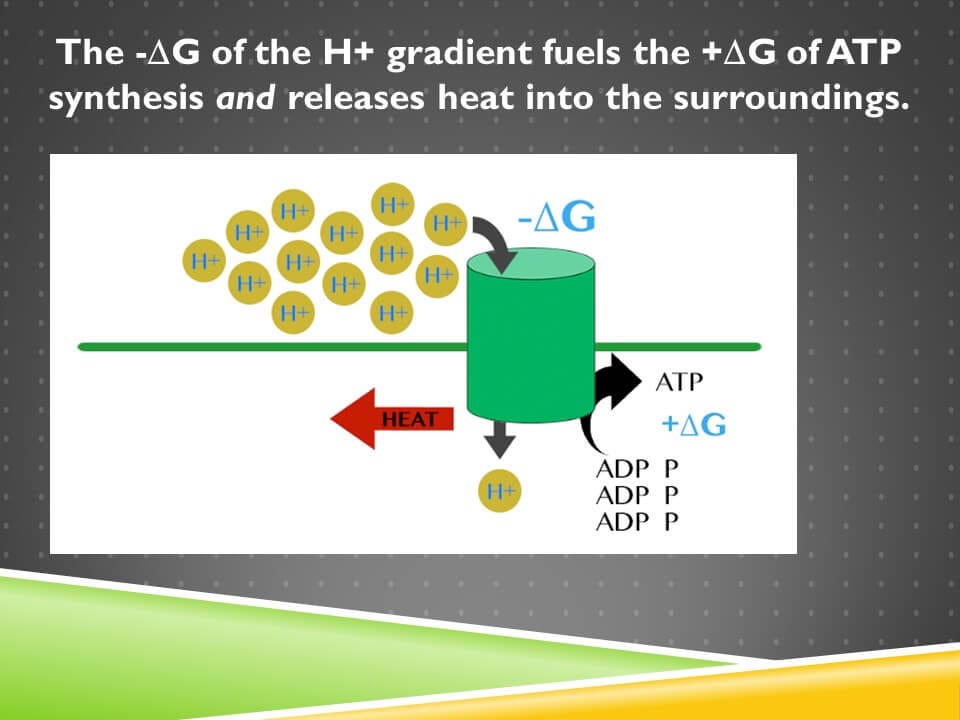
We synthesize ATP in our bodies using a protein called ATP synthase. This protein is depicted in a very simplified form as a green cylinder on the screen. ATP synthase uses the energy stored in a hydrogen ion gradient to synthesize ATP.
Now if you look at the hydrogen ions, they’re all located on one side of the membrane instead of the other. That’s a highly ordered state that took a lot of energy to produce. They want to reach their “equilibrium distribution” where they’ll be equally distributed across the two sides of the membrane.
That never happens because we invest so much energy in concentrating them on the side of the membrane. And that’s very analogous to how we construct a dam, which is like the membrane, to hold water in a high position in the hydropower plant. Only here we have a membrane that’s holding the hydrogen ions in a high concentration on one side of the membrane. Just like the dam allowed the water to release its energy and flow to a lower state, through the intake, through the penstock so it would turn the turbine, these hydrogen ions are going to flow through ATP synthase and turn a turbine inside of it that’s not depicted here.
The kinetic energy of that movement is then transferred to ADP and phosphate and ultimately transferred into the energy stored in the terminal phosphate bond of ATP. There’s a few different kinds of energy that are demonstrated right here.
So, first of all, whenever a particular form of a chemical, a particular chemical species, is all held in a high concentration, we call that a concentration gradient. Furthermore, the hydrogen ions are positively charged. Whenever there’s a concentration of a positive or a negative charge in one area, we call that an electrical gradient. Because we have both of them together in the hydrogen ion gradient, we call that an electrochemical gradient. When they disperse and release their energy, we call that moving along their gradient. In later lessons, we’ll see how we get them in into that side of the membrane in the first place. We do that by investing energy and pumping them across the membrane so they concentrate there, that’s called moving against the electrochemical gradient. The chemical bond energy is what’s stored in ATP. All of these are forms of potential energy, except the movement of the hydrogen ions along their electrochemical gradient, and the turning of the turbine inside ATP, that’s kinetic energy.
And just in the way we saw the hydropower plant take the potential energy of water, convert it into the kinetic energy of its movement, the kinetic energy of the turning of the turbine back into the potential energy stored in the electric cables, we’re seeing the same thing here: potential energy interconverted to other forms of potential energy with kinetic energy as an intermediate.
Video Time: 23 min 00 sec
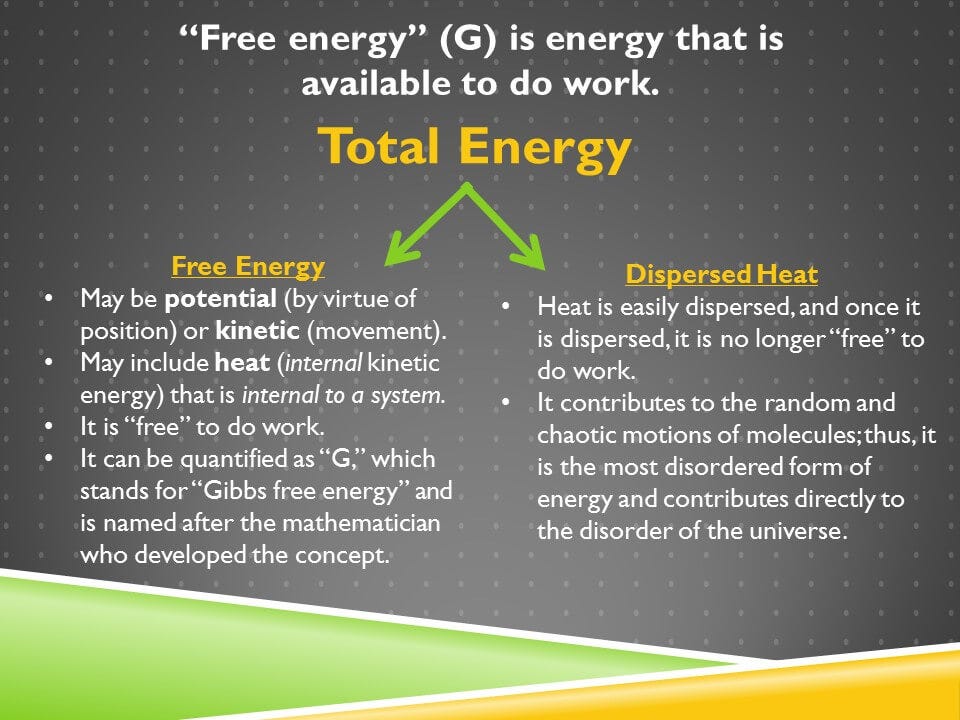
There’s another concept that’s important to introduce here and that’s the concept of free energy. Although the first law of thermodynamics holds that the total energy of the universe is always constant, it also holds that it can change forms. And the concept of free energy allows us to separate energy into forms that are useful to do work and forms that aren’t.
And free energy is energy that’s free to do work. It could be potential energy, energy by virtue of something’s position. It could be kinetic energy, the energy of movement. It could include heat, as long the heat is internal to a system and can be harnessed to do work. An example of that would be when we use heat to do the work of cooking our food.
We can quantify the free energy as G. G stands for Gibbs. Gibbs was one of the pioneers in this field of science and it’s named after him. And we can give a value to G, we can give a number value to G, and a unit value to G. We’re not going to do that here, we’re only going to talk about it at the conceptual level. But energy, especially once it’s converted to heat, is very likely to disperse.
You can turn on your stove and if you don’t start cooking something that energy is not going to hang around waiting to cook your food, it’s going to disperse as heat. And once heat disperses, it disperses to a point where we can’t harness it anymore. Once that heat goes into the atmosphere and into space, good luck getting up there and bringing it back down to earth.
And so once that heat is lost from the system it’s only contributing to the disorder of the universe by making molecules randomly fluctuate. And it’s no longer able to do the work of creating order. And since energy is always going to become heat at some point, some of it’s always going to get dispersed, and that means that we are always losing free energy over time.
So although the first law says the total energy remains constant, the second law says that the free energy, that G, is always decreasing. And in fact the constant decrease in G is the reason that the disorder or entropy of the universe is always increasing.
Video Time: 25 min 35 sec
We can talk about the change in free energy, or G, over time, not only in the sense of a total amount, but also in the sense of specific chemical reactions. The change in G is delta (Δ) G. And the ΔG of the universe is always negative because it’s always decreasing. But the ΔG within a system can be positive, and the ΔG of any given chemical reaction can be positive or negative.
If something has a negative ΔG, that means that free energy is released and lost as dispersed heat. This is something that occurs spontaneously. It involves a decrease in the amount of order, and it’s referred to as energetically favorable.
If something has a positive ΔG, that means that energy is incorporated that will not occur spontaneously. The amount of order increases and we refer to this as energetically unfavorable.
Video Time: 26 min 35 sec
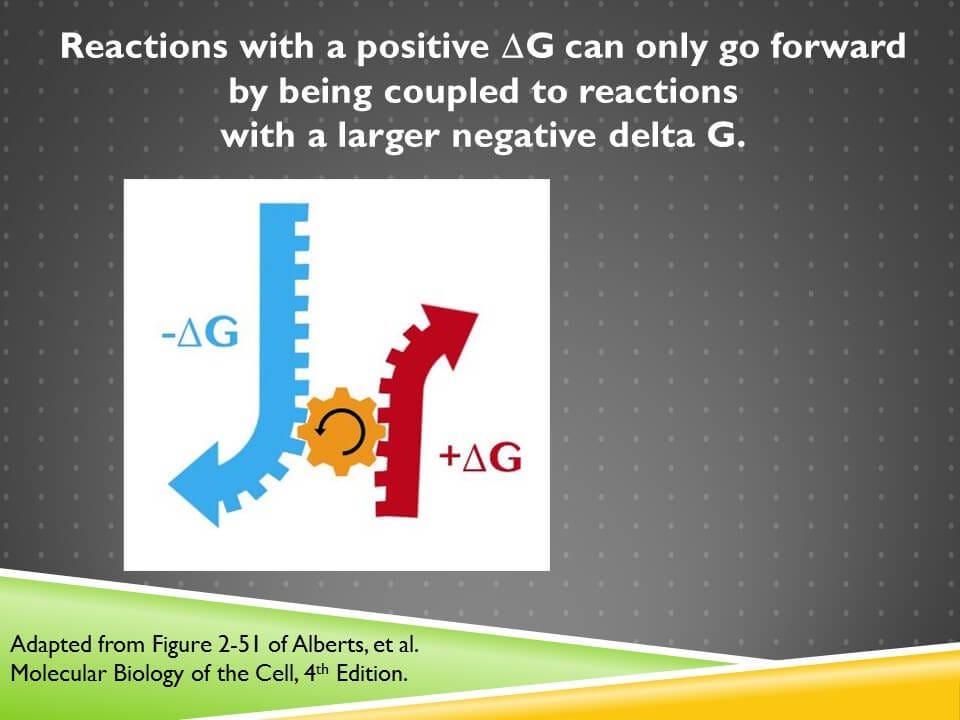
If we’re going to create and sustain order within our bodies, we need a lot of reactions that have positive ΔG’s. But the only way that we can make those reactions go forward, is to couple them to reactions with negative ΔG’s
There’s two reasons for that. One is that the energy has to come from somewhere, so it comes from the reaction with a negative ΔG. But also whenever we have a positive ΔG, we need to more than compensate for it with something that has a larger negative ΔG. And that’s because the disorder of the universe is always increasing, the net ΔG of the universe is always negative, so that math has to add up in that way.
Shown on the screen is one gear turning another. The blue gear represents a reaction with a negative ΔG. It’s providing the energy for the reaction that’s shown in red with a positive ΔG. But the gear is bigger and that’s showing that the magnitude of that negative ΔG has to be larger than the magnitude of a positive ΔG.
Video Time: 27 min 53 sec
If we apply this to ATP synthase, the flowing of the hydrogen ions along their electrochemical gradient has a negative ΔG. The synthesis of ATP has a positive ΔG.
The magnitude of the negative ΔG associated with the movement of hydrogen ions, has to be greater than the magnitude of the positive ΔG of ATP synthesis, because there has to be energy left over to be released as heat and that heat has to cause a greater decrease in the order of the universe and the free energy of the universe than is invested in the synthesis of ATP.
When we couple things with a negative ΔG to things with a positive ΔG, we have to have them structurally connected in a very direct sense. They’ve got to be touching each other.
If these hydrogen ions flew through the membrane somewhere over here (away from ATP synthase), that’s not going to do anything to power the synthesis of ATP. If you turn on your stove and you don’t put any food in there, it’s not going to cook the food in your refrigerator; instead, it’s all going to get lost as heat. And so the exact same principle applies here.
Further Reading
NPR: The Average American Ate (Literally) A Ton This Year
This provides links back to the original data supporting the consumption of one ton of food per year. The perspective is that this is a major problem and a cause of obesity. However, if you simply subtract any reasonable estimate of the caloric excess that would cause the development of obesity over time, you inescapably arrive at the conclusion that we eat our bodyweight in food many times per year and hundreds of times per lifetime.
Catalysis and the Use of Energy by Cells
This is a section of the 4th edition (2002) of Molecular Biology of the Cell, freely available on NCBI bookshelf, that treats energy concepts at approximately the level I treat them in the first two lessons of this class. This book is now in its sixth edition (2014), which is available on Amazon. If you only want to read this section, the free version is fine, as the basic concepts have not changed much. If you really want to study molecular biology, definitely purchase the most recent edition, as much changes in this field over 12 years.
Chapter 20 in Silberberg, Chemistry: The Molecular Nature of Matter and Change
This provides a much more detailed treatment of thermodynamics. If you haven’t studied chemistry yet, you can use this chapter to take the concepts in this lesson to the next level. If you’ve mastered chemistry, you’ll recognize this lesson as a light review of the thermodynamic concepts taught in a general chemistry class.
Section 1 of “How Cells Obtain Energy From Food” in Molecular Biology of the Cell
This compares and contrasts our metabolism with that of an automobile.








Share this post