If you’re having issues with playing the video above, play this instead:
The first lesson in the course is free to everyone.
The antioxidant system is profoundly important to health, yet profoundly misunderstood.
In this "Masterclass With Masterjohn" video series, we look in depth at this system and how it helps drive reactive oxygen species (ROS) such as superoxide and hydrogen peroxide away from their pathological roles and toward their physiological roles.
Lesson one covers the physiological roles, taking as examples the following. In the oxidative burst (respiratory burst), superoxide, hydrogen peroxide, and hypochlorous acid are generated within the phagosome of phagocytes to kill and digest pathogens.
In the thyroid follicle lumen, copious amounts of hydrogen peroxide are used to oxidize ionic iodine to diatomic iodine. In response to excess energy or the demand to produce large amounts of ATP, mitochondria generate more ROS and the ROS help restore energy balance by 1) inhibiting aconitase and shunting citrate into the cytosol where it is used for fatty acid synthesis instead of being used for ATP production, 2) inhibiting glucose uptake and the entry of fatty acids into the mitochondrion for beta-oxidation, 3) increased mitochondrial biogenesis, and 4) increased production of antioxidant defenses.
In the next lesson, we will look at what happens when all this goes wrong: the pathological roles of oxidants.
Listen to the Audio
Listen to the audio here.
Transcript, Slides, References, Further Reading
These materials are free to everyone for lesson 1. Members of the Masterpass have access to transcripts, slides, references, and further reading for the entire course. To learn more about the Masterpass, click here.
Read the transcript and other materials below, or download them here.
Hi. I’m Dr. Chris Masterjohn of ChrisMasterjohnPhD.Com, and you’re watching Masterclass With Masterjohn, where we’re going to take a look at the antioxidant defense system.
This is the first in a series of video lessons where we take apart that system. This is a system where it is helping us recover well from exercise, age gracefully, avoid all kinds of diseases, feel good, look good, so many important things it does for us so it’s really important to understand and yet it’s also so misunderstood.
And if you think about antioxidants, what do you usually think of? I think most of us think of things like fruits and vegetables. That’s all well and good. Things like vitamin C and vitamin E. That’s all well and good. But how often do we think of protein or carbohydrate or B vitamins?
All things that are just as important to the function of this system. And so if we want to be conscious and mindful about how to support our own health we should be thinking about antioxidants and we should understand how the system works so we can think about them correctly.
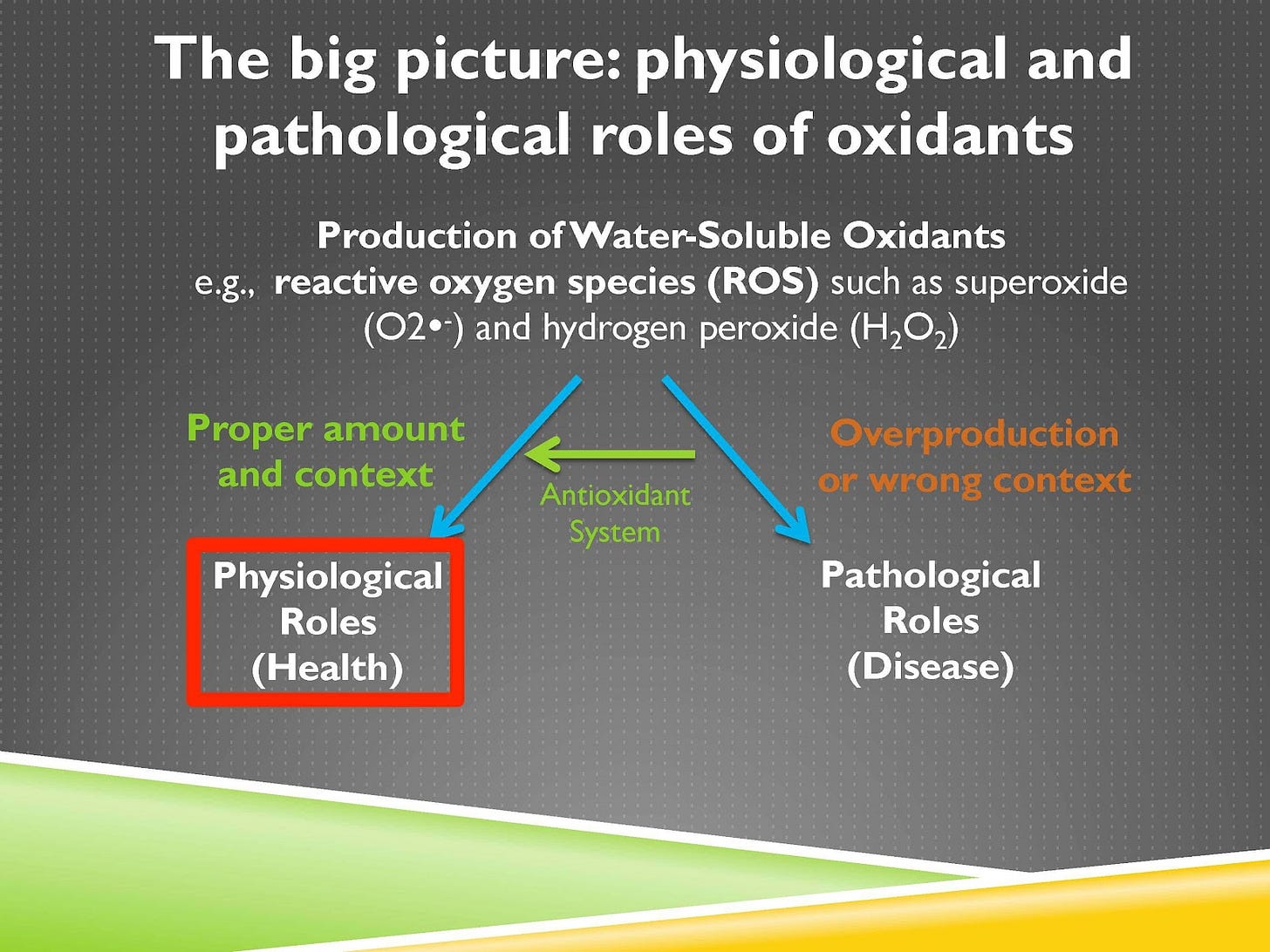
So in this series of lessons we’re first going to start by looking at why the function of the antioxidant system is not simply to prevent the accumulation of oxidants or to counteract them, and it’s not that oxidants are bad and antioxidants are good. Rather oxidants can play important healthful roles in physiology or pathological roles that contribute to disease, and the purpose of the antioxidant system is to direct those oxidants into their physiological healthful roles and away from their pathological disease-causing roles.
Related: Redefining Oxidative Stress
Related: Radical-Free Biology of Oxidative Stress
Video Time: 2:05
That’s depicted on this screen. So you can see the big picture of the first few lessons is that you can have water-soluble oxidants, which is the term I’m often using but we could say reactive oxygen species, some of them are reactive nitrogen species that we’ll talk about, but some prototypical examples of these include superoxide and hydrogen peroxide and when they’re used in the proper amount and context that leads to physiological healthful roles. It’s only when they are either overproduced or produced in the wrong context that that leads to pathological disease-causing roles. The antioxidant system is shifting them from the pathological roles to the physiological roles. And what we’re going to zero in on today is what’s shown in this red box, physiological health-promoting roles of oxidants.
Video Time: 3:00
So this is not comprehensive but just taking a few of the key illustrative examples, the ones we’re going to talk about are in immune system cells we have oxidants killing pathogens. That’s protecting us from infectious diseases. Cleaning up dead and dying cells and the debris of the cells or excess energy.
In the thyroid gland we have hydrogen peroxide oxidizing iodide to iodine, which is necessary for thyroid production. We can’t make thyroid hormone without oxidants. And then in the system of energy metabolism we’ll see oxidants as signals of energy overload that communicate to the cell the need to decrease energy uptake and to increase the capacity to burn energy. And by capacity I mean the ability to burn more energy and to burn that energy more cleanly.
So let’s tackle the first one first.
Related: Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for?
Video Time: 4:10
We can imagine this cell shown on the screen as a phagocyte. A phagocyte is a cell that eats things.
gobble gobble gobble nom nom nom nom nom
And phagocytes are involved in killing pathogens and that’s what we tend to associate the immune system most with. They can also clean up dead or dying cells the debris left over by those cells or even excess energy. So too much fat in your liver, your adipose tissue, immune system phagocytes are often called to provide a number of roles but including cleaning up that excess energy. Now immune system cells for any of these functions need to be able to produce oxidants. And what we’re looking at here is a phagocyte that is not currently eating anything.
So you can imagine this brown object here, which could be that pathogen, or the dead or dying cell, the excess energy, whatever it is that’s the object that could be phagocytosed meaning swallowed up by the cell. But it isn’t yet. And here’s a phagocyte that could be eating and gobbling up that brown thing but it isn’t yet.
In this phagocyte there is something called the NADPH oxidase complex and this is one of the key enzymes involved in producing oxidants. And when the cell is at rest it is separated into a number of different subunits and the subunit picture is actually much more complex than what’s shown here this is simplifying it just to get at the principle that at rest there is a division between the subunits that later comes together when it becomes active.
And so the way it’s shown here there are several subunits that are not associated with the membrane and there is one subunit that is associated with the membrane. This one subunit is the catalytic subunit meaning it’s the subunit that is involved in the active production of the oxidants but it can’t do anything yet because it will only become active when it joins together with these other subunits of the complex.
Video Time: 6:42
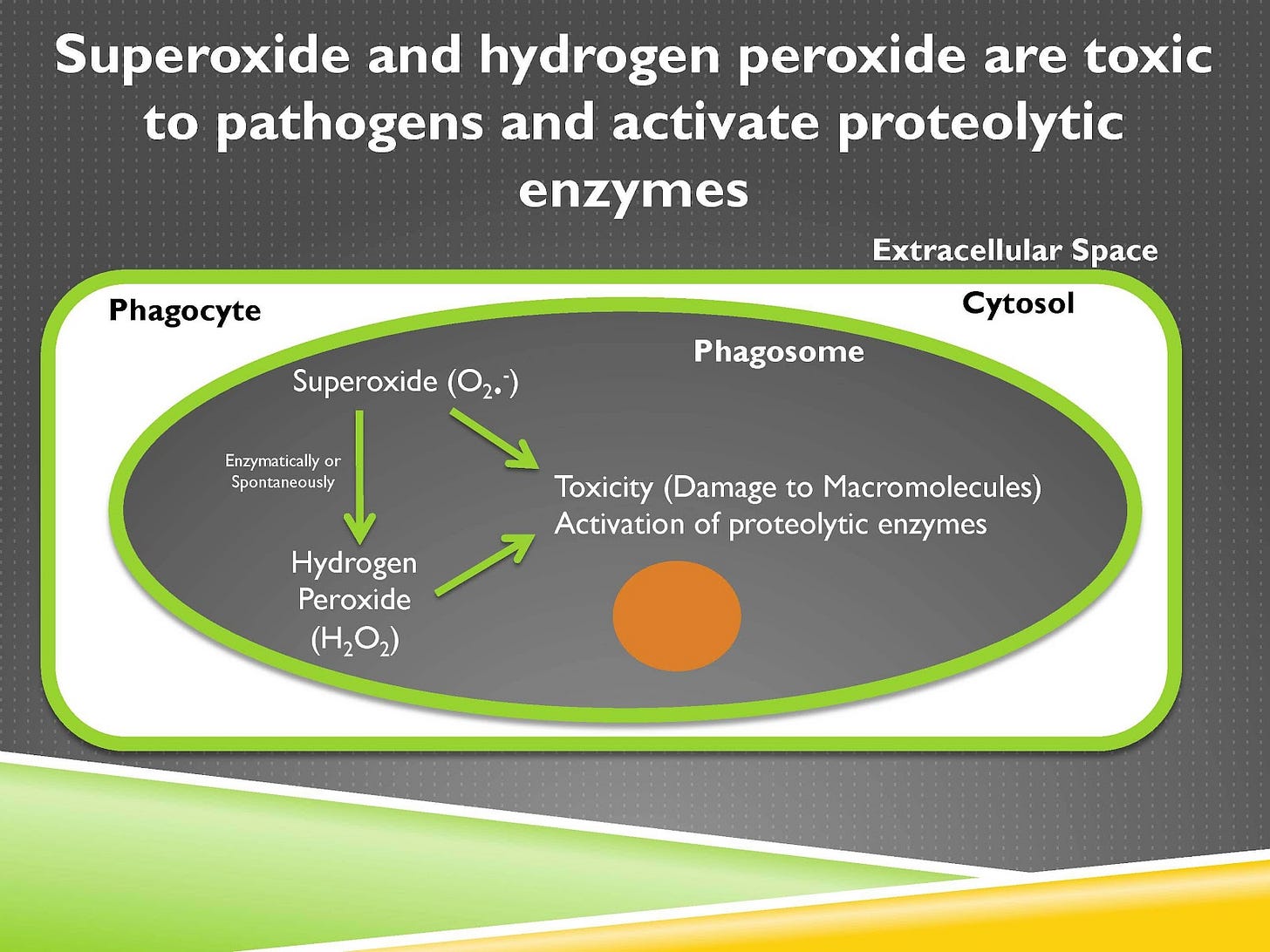
Now when this phagocyte starts eating this object it invaginates its membrane like so and this invagination is an area that will eventually pinch off right here and when it does so it will have a new space that is completely enclosed within it and we will now call that a new organelle called the phagosome. And inside the phagosome the phagocyte has as its goal to put lots of oxidants and enzymes that will break down and destroy, and if it’s alive kill, whatever that object that had been phagocytosed.
So let’s just say this is an example of a pathogen. If this brown pathogen in the center of the phagosome is a threat to your body you want to kill it so you’re going to produce lots of oxidants that can kill it, lots of enzymes that can just utterly destroy it.
Now the phagocyte has a problem, which is if it’s producing tons of toxic dangerous things then it’s going to digest itself or destroy itself or kill itself. So isolating all of this in the phagosome is critically important. When that invagination happens you have the subunits of the NADPH oxidase complex coming together and that activates the catalytic subunit. And that catalytic subunit is going to take oxygen and add electrons to it.
Those electrons come from NADPH, that’s why it’s called the NADPH oxidase complex. NADPH is not shown here, but it’s carrying those electrons from glucose. Now when it adds electrons to oxygen, oxygen becomes superoxide. Superoxide, signified by this dot, is a free radical. That means it has an unpaired electron, and that gives it a voracious appetite to get another electron to pair them up, and that makes it highly reactive.
This formation of superoxide and the oxidants made from that we’ll talk about soon is called the respiratory burst or the oxidative burst, and it’s a central feature of the immune response. Now we can take this superoxide and we can do a couple things with it.
Video Time: 9:17
First of all we can make hydrogen peroxide from it. You may have hydrogen peroxide somewhere in one of your cabinets and maybe you use that when you’re wounded to kill pathogens. Our immune system is doing the same thing. Superoxide and hydrogen peroxide are toxic and can damage macromolecules, meaning large structures within that pathogen. And they can also activate proteolytic enzymes, which are enzymes that digest protein.
So that means that they have both the capacity to kill the pathogen and also to activate its digestion.
Video Time: 10:00
The end consequence of this is that the pathogen is destroyed and that is a successful immune response.
Related: Myeloperoxidase in human host defense.
Video Time: 10:12
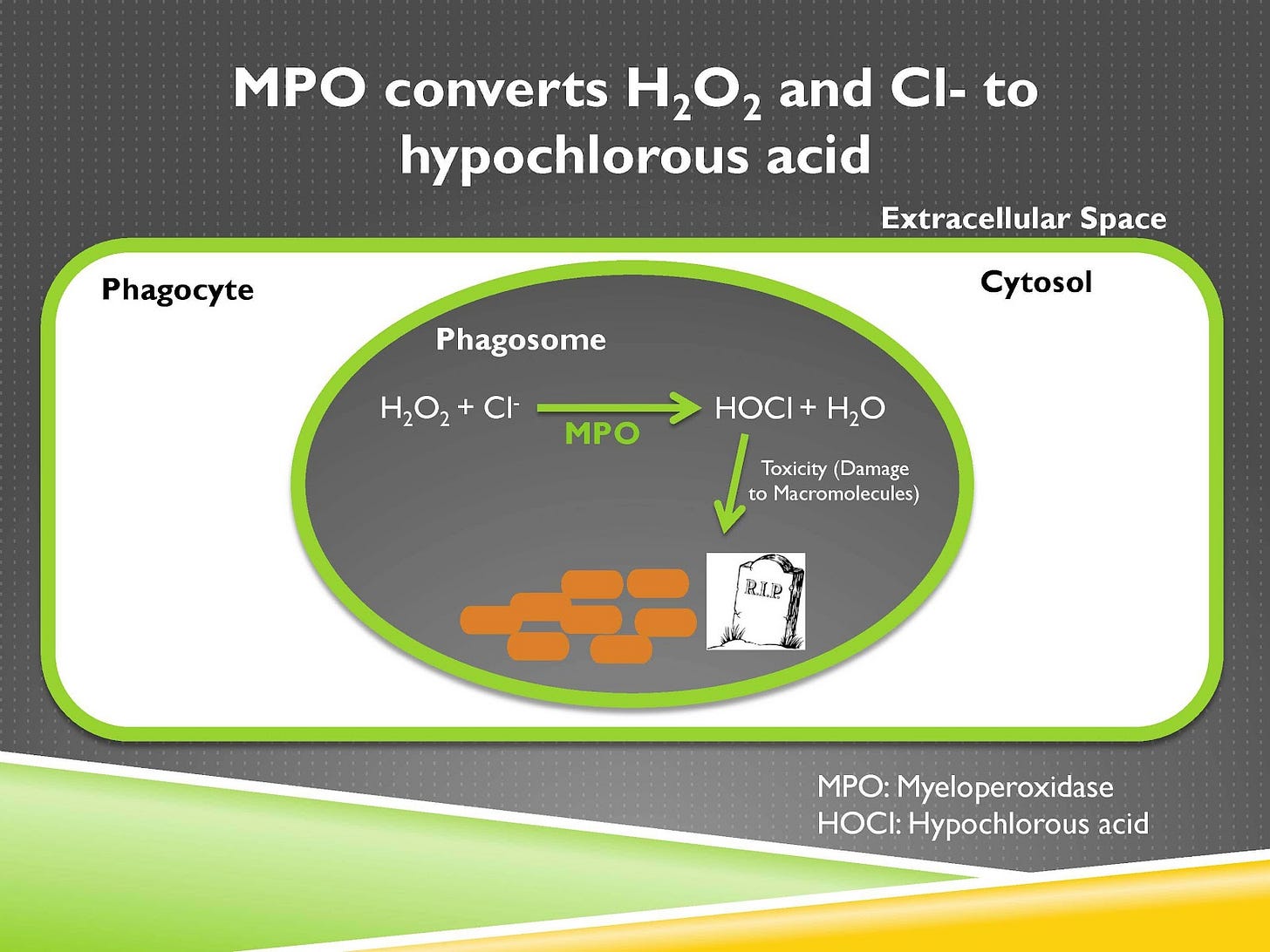
A further role of these oxidants is for an enzyme called myeloperoxidase, which is stored within granules to fuse with the phagosome and when it does so that enzyme will be released into the phagosome and will convert hydrogen peroxide to hypochlorous acid, otherwise known as bleach.
Video Time: 10:28
So if you’ve used bleach to sanitize countertops in a laboratory or kitchen or bathroom or to sanitize the floors or whatever else, you’re using it to kill pathogens, our immune systems are making bleach inside our own bodies for that very purpose.
Video Time: 11:03
The enzyme myeloperoxidase that’s responsible for making the bleach has a green color and so if you ever experienced your snot or phlegm becoming greenish in color when you’re sick that is reflective of MPO.
MPO, myeloperoxidase, that bleach-producing enzyme, is what’s giving your phlegm or your slot the green color. So that’s a few examples of the use of oxidants in the immune system, let us move on to the thyroid gland.
Video Time: 11:38
Related: Mechanism of hydrogen peroxide formation catalyzed by NADPH oxidase in thyroid plasma membrane.
The thyroid gland has an analogous system of using oxidants but for a very different purpose. The thyroid gland’s “goal,” so to speak, is to take hydrogen peroxide and oxidize iodide to iodine. You can see in the picture here iodide is the ionic form of iodine. “Ionic” means it has a negative charge and also means each iodine ion is floating around individually.
During its oxidation it becomes diatomic iodine. This is a molecule composed of two iodine atoms joined together, that’s why it shows “I2” and that is what is used as the precursor to thyroid hormone. And the way that you oxidize iodide to iodine in the thyroid is with the enzyme thyroid peroxidase or TPO and the oxidant used for that purpose is hydrogen peroxide.
Now that means that the thyroid has to produce tons of hydrogen peroxide to get this done but we already saw in the case of the phagosome that we had to isolate that hydrogen peroxide somewhere, namely in that case in the phagosome.
Why? Because just as it’s toxic to the pathogens it’s also toxic to the phagocyte or to the surrounding tissues and we don’t want to damage our thyroid gland either so the thyroid gland needs to have a way to compartmentalize the hydrogen peroxide.
The way it does that is that it has a dedicated space called the follicle lumen and this is within the thyroid gland but it is outside of the thyroid cells. So the main form of cell in the thyroid gland is the thyrocyte and everything within this green compartment here is part of the thyrocyte.
The thyrocyte has NADPH oxidase inside of it and also has it seated at the membrane, and hydrogen peroxide can get produced at both places and the hydrogen peroxide produced here is meant to go outside the cell and here it is actually being produced outside the cell.
In both cases we want the hydrogen peroxide to accumulate in the follicle lumen and we want to minimize its accumulation inside the thyrocyte. Oxidizing iodide to iodine outside of the thyrocyte helps keep all of the oxidants out there where they’re not going to damage the cells of the thyroid gland.
So in these cases we are kind of alluding to what we’ll see in future lessons where what we want for these oxidants is for them not to be produced we don’t want to say we want to make less we want to say that we want to make the right amount and put it in the right place at the right time. So at the beginning we said the proper amount and context, that context is where, and that context is when.
In the case of the phagocyte, where is in the phagosome. When is when you need them because you’ve just eaten up a pathogen or something else you want to destroy. In the thyroid gland, where is in the follicle lumen, and when is when you need to make thyroid hormone.
Together, where and when is context.
If where and when is good, physiological healthful roles of oxidants.
If where and when is bad, that’s when you get a problem.
Okay now let’s take another example in energy metabolism.
So in order to explain the role of oxidants in energy metabolism, we need to back up a little bit and explain a few principles of how we regulate energy metabolism in general.
Related: Citrate Carries Acetyl Groups from Mitochondria to Cytosol for Fatty Acid Synthesis, corresponding to p. 666 in Berg, Biochemistry
Video Time: 16:00
So first of all if you we’re breaking down energy in order to make ATP, which is the universal energy currency of the cell, we need ways to know do we want to break down more, or not? And there are two reasons to answer “no” to that question. One is that we don’t need any ATP, and that’s another topic that were not covering in this lesson.
Reason number two is we can’t handle the demands placed on the mitochondria to make that ATP so we have to say hold up, the mitochondrion is saying, “I can’t handle the tasks given to me so do something else with this energy.”
One of the consequences of that is that when the mitochondrion can’t handle more energy it’s going to say, “take this energy and store it as fat.”
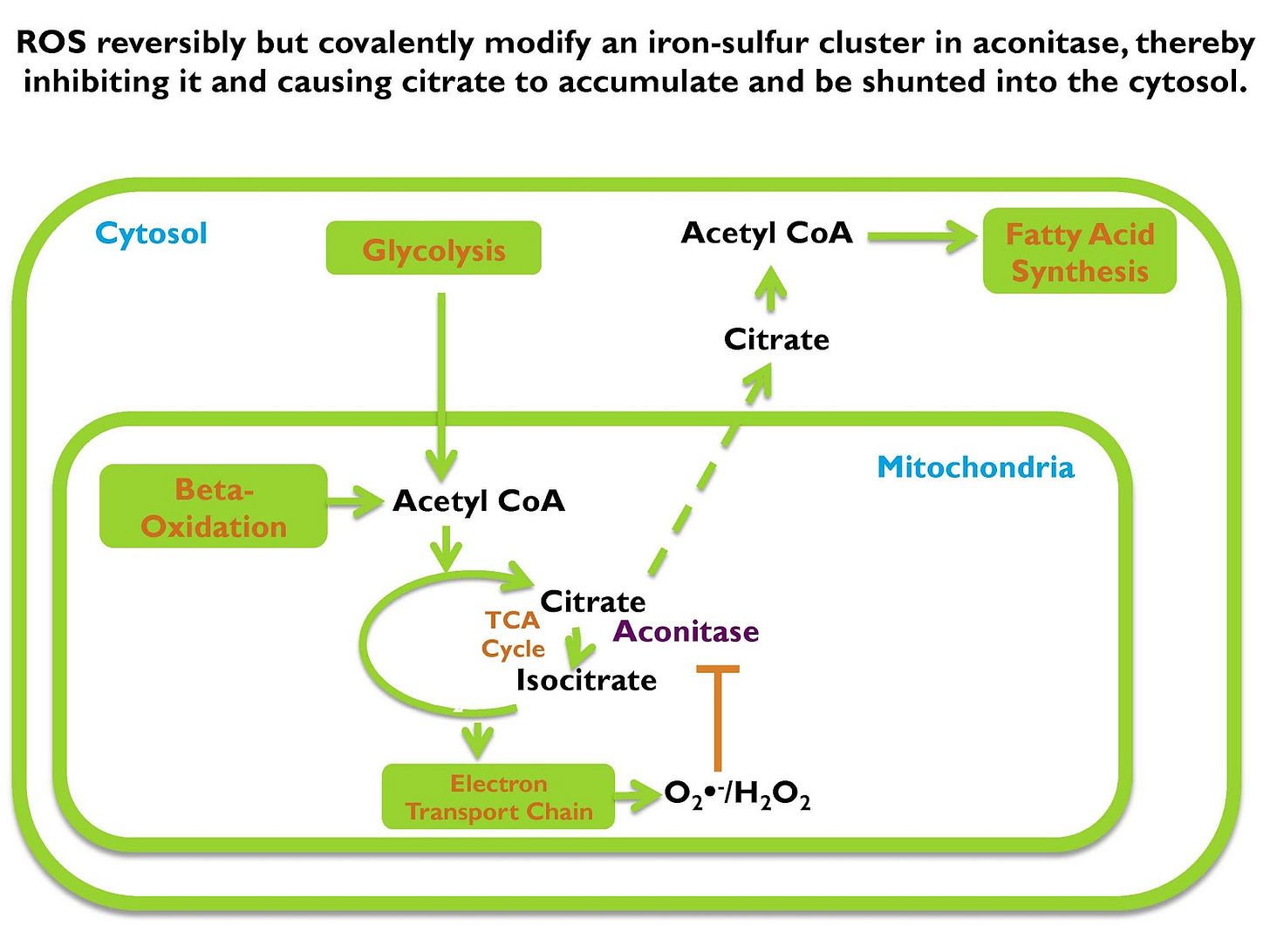
Now, shown on the screen is the critical biochemical event that initiates that decision to store the energy as fat. If we take acetyl CoA, which is the intersection of all anabolic and catabolic reactions, meaning all breaking down things and building them up, usually if we make fatty acids, for example, we make them from acetyl CoA. If we burn fatty acids for energy, we break them down to acetyl CoA.
We can get acetyl CoA from carbohydrate, from fat, from protein, doesn’t matter. Shown in the picture is, it’s depicting it as getting it from glycolysis, which is burning carbohydrates for energy, or beta-oxidation, which is burning fat for energy, doesn’t matter where it came from, it’s acetyl CoA.
Acetyl CoA is going to enter the TCA cycle, which is how it’s further broken down for energy and if we’re going to break it down fully so we can maximize ATP yield, we’re gonna do that in the electron transport chain. But before we get to the electron transport chain we enter the TCA cycle and the first thing that’s going to form is citrate.
Now if the cell decides “I’m going to metabolize citrate to fully break it down for ATP,” it continues to go through more steps in the cycle, but if the cell says “I’m not going to break down more energy, I’m going to store this as fat, it shunts citrate from the mitochondrion into the cytosol which is the main water-based fluid in between all the cell’s organelles, and in that cytosol, that citrate generates acetyl CoA, and that acetyl CoA is used to synthesize fat.
Now that means that citrate moving from the mitochondrion to the cytosol is the critical biochemical event that initiates fat storage.
How do oxidants regulate this picture?
Related: Redox-dependent modulation of aconitase activity in intact mitochondria.
Video Time: 19:10
Well let’s say we’re breaking these down for energy in the electron transport chain.
The electric transport chain most of what it does is it takes oxygen and it makes water. And the process of taking oxygen and turning it into water is what allows it to engage in many other reactions that help us extract energy from food to make ATP. But a small amount of oxygen is always used to make superoxide and hydrogen peroxide, oxidants that we’ve been seeing from the beginning.
If you place more demand on the mitochondrion than it can handle, if you’re saying, “Here take all this energy and make ATP” and the mitochondria can’t handle all that energy, the concentration of superoxide and hydrogen peroxide increases. And as it increases, it traverses into the TCA cycle where it will inhibit the enzyme aconitase.
Aconitase is the enzyme that converts citrate to isocitrate and that’s what allows citrate to go further down in the cycle to be fully burned for energy. Aconitase has an iron-sulfur cluster that gets oxidized by superoxide and hydrogen peroxide and if it gets oxidized it gets inhibited.
If you inhibit aconitase, you inhibit the conversion of citrate to isocitrate. That causes citrate to get backed up in the mitochondrion. When citrate accumulates in the mitochondrion, it moves into the cytosol. When it moves into the cytosol it becomes the source of cytosolic acetyl CoA, which is used as the substrate for fatty acid synthesis.
So if you have incoming acetyl CoA from carbohydrate, from fat, from protein, from wherever, and it goes to the TCA cycle. If the mitochondrion can handle taking in more citrate and metabolizing it fully, breaking it down for energy making ATP, it will do so. But if it can’t, it makes more and more superoxide that gets converted to hydrogen peroxide and those oxidants inhibit aconitase, cause citrate to accumulate, travel into the cytosol and be used for fatty acid synthesis.
So reactive oxygen species are communicating excess demand on the mitochondrion, they cause energy to be diverted into fat storage. Now if diverting energy to fat storage relieves that burden on the mitochondrion enough that reactive oxygen species go back down to normal, all well and good.
But what if it doesn’t?
The cell has numerous other ways that it responds to the increased oxidant burden.
Related: Voltage-dependent anion channel (VDAC) as mitochondrial governator—Thinking outside the box
Video Time: 22:04
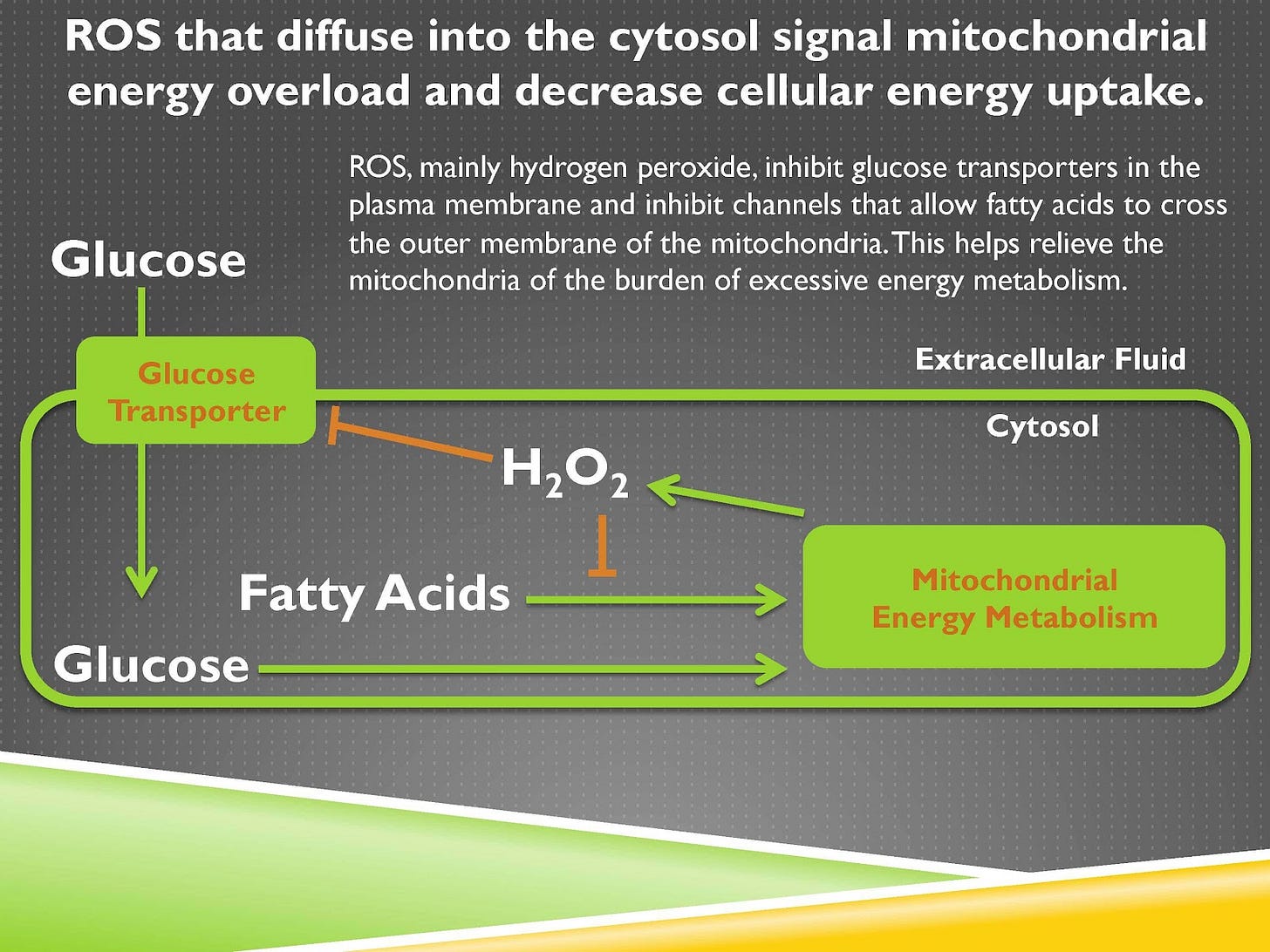
First of all, hydrogen peroxide can diffuse into the cytosol where it will inhibit glucose transporters. That inhibits glucose entering the cell. It can also inhibit fatty acids from entering the mitochondria. So it’s acting to inhibit the incorporation of any new energy.
So, step one is take whatever energy you have, direct it towards fat storage. Step two is stop taking in new energy.
Video Time: 22:40
It also acts on the nucleus and the mitochondrion to increase the expression of genes involved in mitochondrial biogenesis, which is the synthesis of new mitochondria, and antioxidant defense. That means being able to burn more energy because of more mitochondria and to be able to burn that energy more cleanly because of increased antioxidant defense.
This is one of the ways that you get fitness from exercise. You exercise, that creates, as that mitochondria becomes more active, more demand is placed on it, it makes more reactive oxygen species, the hydrogen peroxide increases the production of more mitochondria, that makes you get fit.
So together then these are all multiple strategies designed to restore the balance between energy that comes in and what the cell can handle for burning energy.
Video Time: 23:45
If we were to sum them altogether, when we have more energy coming in as shown here on the left, if that’s overwhelming the capacity of the mitochondria to burn that energy with increased production of oxidants from the mitochondria and they’re going to increase fatty acid synthesis, decrease glucose uptake or fatty acid uptake into the mitochondria, increase the production of new mitochondria and antioxidant defense.
As you can see clearly this is a physiological adaptive response because it’s helping the mitochondria adapt to the demands placed on it.
All right! So we’ve looked at physiological healthful roles in the immune system, the thyroid gland, and energy balance. This is not comprehensive, it’s just a few illustrative examples.
Next time what we’re going to talk about is when all these things go wrong when we can have pathological roles of oxidants. But that’s all for today signing off this is Chris Masterjohn of ChrisMasterjohnPhD.Com, and you been watching Masterclass With Masterjohn.
Further Reading
Dean Jones. Redefining Oxidative Stress. 2006.
Dean Jones. Radical-Free Biology of Oxidative Stress. 2008.
Bylund.Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for? 2010.
Nauseef. Myeloperoxidase in human host defense. 2014.
Dupuy.Mechanism of hydrogen peroxide formation catalyzed by NADPH oxidase in thyroid plasma membrane. 1991.
Citrate Carries Acetyl Groups from Mitochondria to Cytosol for Fatty Acid Synthesis, corresponding to p. 666 in Berg, Biochemistry
Bulteau. Redox-dependent modulation of aconitase activity in intact mitochondria. 2003.
Lemasters. Voltage-dependent anion channel (VDAC) as mitochondrial governator—Thinking outside the box. 2006.















Share this post