In my Cholesterol Podcast on the Livin' La Vida Low-Carb Show with Jimmy Moore, I offered the view that atherosclerosis and many other degenerative diseases can be seen as a process of oxidative damage wherein polyunsaturated fatty acids (PUFAs) get damaged by toxins, heavy metals, and byproducts of normal metabolism, break into pieces, and then continue to damage other molecules. I suggested thinking of this as breaking a glass on the floor — the glass breaks into shards, and then becomes dangerous. Step on the shards, and your foot will bleed. Likewise, when proteins, DNA, and other important molecules come into contact with “the pieces of broken PUFAs” they get seriously hurt.
PUFAs are not the only molecules subject to this type of damage. “Oxidative stress” is very similar to and very related to “nitrative stress” and “carbonyl stress.” “Carbonyl stress” involves the breakdown products of sugars.When our bodies metabolize sugar, we first do two things to it: first, we add some phosphate to it in order to trap it in the cell, target it through a specific series of reactions, and provide some of the energy necessary for those reactions; second, we split it in half. The technical term for this process is “glycolysis.”
A small percentage of the resulting half-sugar will spontaneously lose its phosphate and degenerate into a compound called “methylglyoxal.”
The next few paragraphs might make your eyes glaze over if you aren't in love with biochemistry. If that happens, you can skip to the last two paragraphs when the plain English will resume.
In the picture of methylglyoxal above, “C” represents carbon, “O” represents oxygen, “H” represents hydrogen, and the lines represent chemical bonds. The carbons double-bonded to oxygens are called “carbonyl groups.” These carbonyl groups are highly reactive. They especially love to react with nitrogens from the “amino groups” of other molecules and form what's called an imidazole ring.
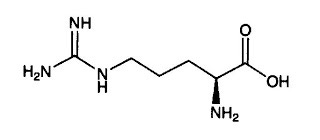
In the picture of the imidazole ring above, “N” represents nitrogen and each corner of the ring structure represents a carbon bonded to a hydrogen. The circle in the middle indicates that some of the electrons travel around continuously through the ring rather than sticking with particular atoms. Imidazole rings are naturally present in the amino acid histidine, but methylglyoxal can form them by spontaneously reacting with the free amino groups of the amino acids lysine and arginine. In doing so, it can damage the structure and function of the proteins these amino acids are part of
Every amino acid has a nitrogen-containing amino group, but on most amino acids this amino group would be bound to other amino acids and tucked away safely within a protein. In the two pictures that follow, this “safe” amino group is the one on the far right. The “side chains” of the amino acids are not always tucked away and are thus not always safe. In the two pictures that follow, the side chains proceed to the left.
The side chain of arginine has two nitrogens, and can thus provide the two nitrogens necessary to form an imidazole ring:
The side chain of lysine has only one nitrogen, so two lysines are necessary to form an imidazole ring:
Consequently, methylglyoxal reacts more often with arginine, by simply creating an imidazole ring in its sidechain that does not belong. This deranged form of arginine accumulates in the body with age and has been found in high amounts in human lens tissue.
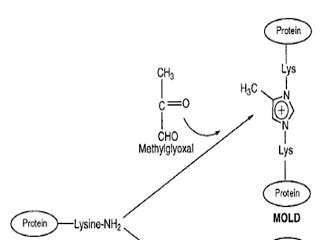
When methylglyoxal reacts with lysine, however, it does something more dramatic. Since two lysines need to participate to form an imidazole ring, lysines that would ordinarily be distant from each other within a protein, or even lysines from two completely independent proteins, can get stuck together. In the first case the shape of the protein could get damaged and in the second case the protein would get stuck to another protein. Think “bumper cars.” It's hard to drive your car when its stuck up against another car — makes a fun game from time to time but if you're playing “bumper cars” on the way to work you're going to get a ticket, your boss isn't going to be happy when you arrive late, and you could get seriously hurt.As you can see in the following picture, two lysines supply “amino” (NH2) groups that interact with the “carbonyl” (C=O) groups of methylglyoxal. The hydrogens (H) and oxygens (O) leave the scene as water. An imidazole ring is formed, leading to an “imidazolium” crosslink between two lysines, whether of the same protein or of two wholly different proteins. The resulting advance glycation endproduct (AGE) is not so affectionately termed “MOLD,” (MethylglyOxal-Lysine Dimer).
And now, returning to plain English…
Like PUFAs, sugars can be broken into harmful pieces, and like shards of glass, they too can damage other molecules. Some authors have suggested that methylglyoxal can, in small amounts, play important physiological functions. Most look at its ability to damage proteins and cause crosslinks within and between proteins and see a molecule running awry, contributing to “carbonyl stress” just like oxidizing compounds contribute to “oxidative stress.”
Over the next several months, I will be performing several experiments to determine what type of dietary factors inrease and decrease the formation of methylglyoxal. I will also be reviewing the literature to better understand what kind of harm, and perhaps, what kind of good, it might do in the body. I will be sure to update you here, so stay tuned!