When Fat Burns In the Flame of Lean Muscle Mass — Better Put That Butter Either on Steak or Potatoes
My last post was political and got 17 comments just in the first six hours. I suppose that means I'm due for another post about religion, or one about sex. Given Stephan Guyenet's recent post about the dangers of hyperpalatability, though, I'm inclined to obey the proverb “don't take too much honey” and delicately sprinkle those sweet and enticing posts on the nourishing bulk of the usual biochemistry you get at this blog.
I try to make this blog more like wine than grape juice — you know, the adult flavors people enjoy in France where obesity rates are lower.
And besides, this post will answer the cliff-hanger I dropped at the end of Monday's post, “Let Us Honor Ancel Keys, Our Patron, As We Cherry Pick Studies to Bash Fructose.”
Peter Dobromylskyj over at Hyperlipid recently posted “Diabetic nephropathy and the lost Swede,” wherein he discussed the ability of a ~95% fat diet to produce weight loss in mice.
In this post, I'd like to take a look at what happened to food intake, hormones, and body composition in that study, and explain why eating butter with no steak, bread, vegetables, or potatoes under it isn't a very good idea.
And no, this is not just due to the “problems of traction presented by the butter-butter interface.” Though I admit that's a problem too.
Here's a link to the study for anyone who wants to follow the reference:
The authors compared a standard chow diet to a “high-fat” diet containing 45% fat and 17.5% sucrose, and to a zero-carb, 95% fat “ketogenic” diet.
They also put one group of mice on calorie restriction, where the mice ate only two thirds of their normal calorie intake. The authors don't describe this diet as clearly but it was apparently a chow diet formulated to give the mice the same amounts of vitamins and minerals they would have gotten on any of the other diets.
The authors reported food intake rather strangely, reporting only day one, day 25, and the total over the first half of the study, stating only that the pattern was consistent in the second half of the study.
Nevertheless, it appears from what they reported that there was no difference in food intake but that at the very beginning of the study mice on the “high-fat” diet ate twice as much food as the others. This is a sign that the diet was more palatable than the others. This supports the idea that sugar makes a high-fat diet extra-palatable, but also shows that a 95% fat diet is palatable enough for a mouse to eat normal amounts of it.
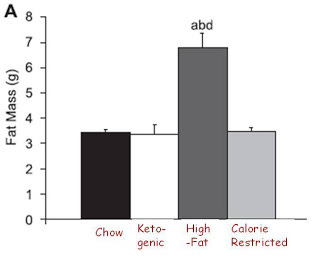
Now let's look at obesity:
As always, you can click on the image to enlarge it.
Interestingly, the only diet that produced obesity was the extra-palatable diet, the one that produced a doubling of food intake at the very beginning of the study but no difference in food intake over the long-term. Although I personally have some data of my own suggesting that palatable diets do not always produce obesity, this observation is nevertheless consistent with the idea that palatability may have some effect on obesity independent of caloric intake. Perhaps Stephan Guyenet's new series on palatability will make some sense out of this.
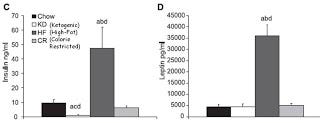
Now let's take a look at insulin and leptin:
Which graph corresponds to fat mass? Clearly, the leptin graph and not the insulin graph. Only the mice who got obese had dramatically elevated insulin, but the ketogenic mice had 90 percent lower insulin compared to chow control mice and no difference in fat mass.
As I wrote late last November in “Is Insulin Resistance Really Making Us Fat?”, leptin resistance is much more closely associated with obesity than insulin resistance is. LIRKO mice have no functional insulin receptors in their liver and consequently have 7-fold higher fasting insulin and 23-fold higher insulin after a meal yet are at least as sensitive to leptin as healthy controls and if anything slightly leaner. Mice and rats with defects in leptin signaling are all insulin resistant and fat.
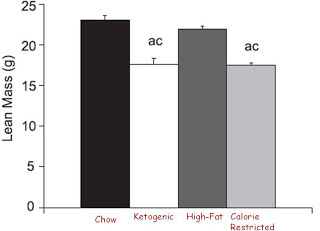
But uh-oh, let's look at lean mass:
Hmm, it looks like eating a 95% fat diet is sort of like starving yourself. All of the weight lost on the super-ketogenic diet was lost as lean mass. And this isn't just because these young mice failed to grow normally; they actually lost weight.
The ketogenic diet wasn't entirely like the calorie-restricted diet. The calorie-restricted mice had lower testosterone, whereas the ketogenic mice had normal testosterone. The mechanism of weight loss seemed different. The ketogenic mice turned more energy into heat than all the other mice.
The authors concluded that the loss of lean mass was not due to muscle wasting because there was no decrease in lean mass in an isolated hind limb like there was in the whole animal. Regardless of whether the loss of lean mass came from their internal organs, bones, hind limb muscles, or some other set of muscles, they still lost lean mass without losing any fat.
There is evidence that the elevated levels of free fatty acids that occurs on an extreme ketogenic diet helps divert energy towards heat production, at least in rodents. But why would this result in lower lean mass instead of fat mass?
In 1895, a biochemist by the name of Rosenfeld coined the expression “fat burns in the flame of carbohydrate.” This was based on observations that cells could break down fatty acids into ketone bodies but without sufficient glucose the cells could not break them down fully into carbon dioxide and hydrogen.
The aphorism offered a simple explanation for why ketones are elevated to extreme levels in diabetes: diabetics do not use glucose efficiently, so glucose levels in the blood rise; since their cells are starved of glucose, the fat stores of these diabetics release their fatty acids and their livers break the fatty acids down into ketones, but these ketones cannot be used in the absence of glucose, so ketone levels in the blood rise and then the ketones are finally lost in the urine. Thus the diabetic is effectively in a constant state of starvation.
We now know that fat burns in the flame of oxaloacetate, which can be derived from either glucose or amino acids.
When we break down fats or carbohydrates for energy, we turn them into acetic acid, or acetate, which is a two-carbon unit. A little shuttle called coenzyme A, which is made of pantothenic acid, carries the acetate around and together we call the complex acetyl CoA. Pantothenate is also called vitamin B5 and is found abundantly in many foods but liver and egg yolks are among the highest (along with certain mushrooms, seeds, and yeast).
In order to fully harvest energy from acetate, we need to send it through the citric acid cycle, also called the Krebs cycle or the tricarboxylic acid (TCA cycle). This cycle will break the acetate down into carbon dioxide and hydrogen. In doing so, it will also release high-energy electrons whose energy can then be harvested to synthesize ATP, a major usable energy currency of the cell. Entry into this cycle is dependent on a compound called oxaloacetate.
Image hosted by Library.Thinkquest.Org
In the presence of glucose, we convert glucose to oxaloacetate. Thus, as oxaloacetate leaves the Krebs cycle cuz it's got things to do and people to see, we can just use glucose to replenish it. In the absence of glucose, we do the opposite: we turn oxaloacetate into glucose. Thus, oxaloacetate gets depleted in the absence of glucose unless we have some other source of it. We can make oxaloacetate from a variety of amino acids, but not from fats. Thus, in the absence of dietary protein or carbohydrate, the only place to get oxaloacetate is to dig into the lean proteins found in our muscles and internal organs.
The authors of the mouse study didn't investigate this explanation, but it seems the most reasonable to me.
Thus, eating an all-fat diet isn't very smart. Of course, virtually no one in the real world would actually do that, at least not at the extreme level used in this mouse study (95% of calories from fat). Hyper-ketogenic diets used to treat disorders of the central nervous system, especially seizures, might get close at around 90% of calories. In any case, biochemistry varies from person to person, and someone attempting a very low-carbohyrate diet who feels fatigued or is losing lean muscle mass might want to consider whether they should up their protein or carbohydrate content.
One thing I really like about The Perfect Health Diet is that although the authors advocate a low-carb diet, they devote a lot of attention to the body's need for glucose, rather than coming up with some silly aphorism like “there are essential amino acids and essential fatty acids, but there is no essential carbohydrate.” The body may be able to survive without dietary glucose, but only because it can make glucose from protein. Give it only fat, and it will make that glucose — and oxaloacetate — from lean muscle tissue.
Better get a steak or potato to go with that butter!