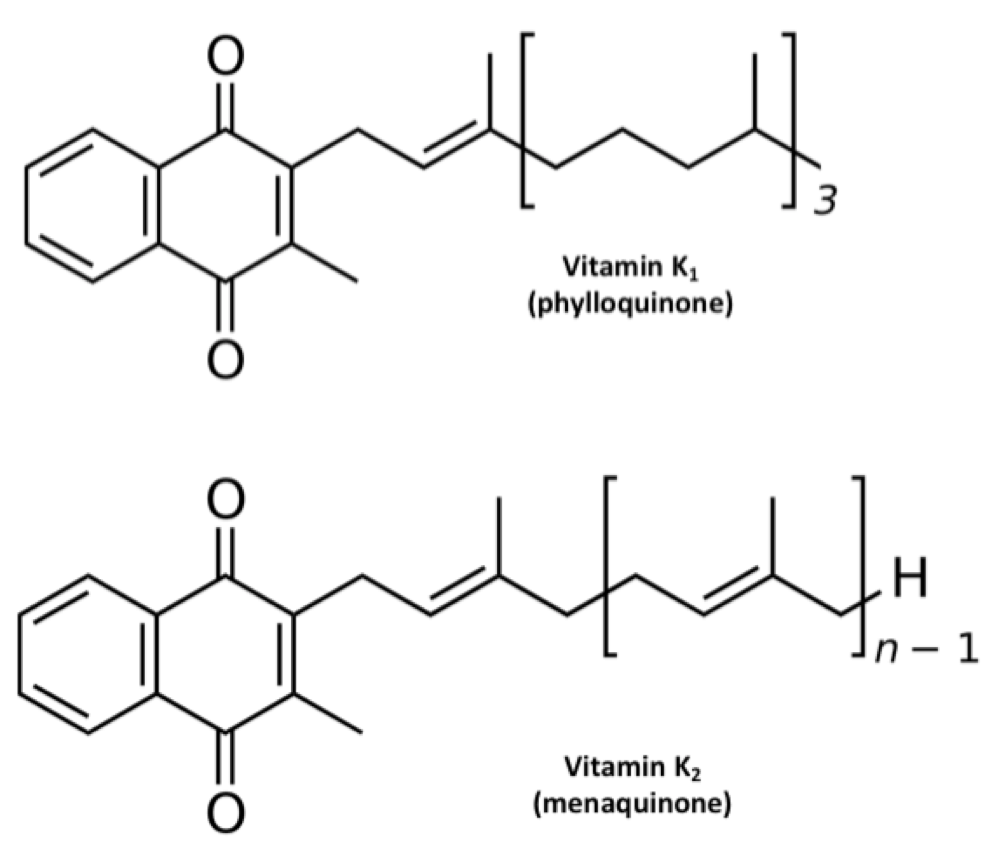
The names “vitamin K1” and “vitamin K2” are artifacts of history (Suttie, 2014). The first form of vitamin K was found in alfalfa, so it was named K1. The second form was found in rotten fish, so it was named K2. As shown in the figure below, they both have the same ring structure, but different tail structures. The tail structures are known formally as side chains. Vitamin K1, now known as phylloquinone, has a mostly saturated tail. Vitamin K2, now known as menaquinone, has an unsaturated tail. Menaquinones are actually a class of compounds with varying tail lengths, designated MK-n, where “n” indicates the number of repeating units in the tail. The specific form of vitamin K2 found in rotten fish was MK-7. When later MKs were discovered, they all had unsaturated tails, so scientists classified them as subforms of vitamin K2.
We now know that this is overly simplistic (Shearer, 2014). Some bacteria, such as those used to make Jarlsberg cheese, produce partially saturated menaquinones wherein some of the repeating subunits have double bonds and others don’t. For example, Jarlsberg is very rich in tetrahydromenaquinone-9, which is similar in structure to MK-9 except the second and third units of the tail are saturated. As Shearer (2014) pointed out, even phylloquinone has a double bond in the first unit of its tail and could be seen as a partially saturated form of MK-4. Thus, rather than forming two categories of K vitamins, it makes more sense to say that vitamin K comes in a wide diversity of forms that are distinguished by the length and saturation of their tails.
Side Chain Length and Saturation Determines Tissue Distribution
While the ring structure is what allows vitamin K to support the vitamin K carboxylase, the enzyme that activates vitamin K-dependent proteins, the tail structure determines how different forms of vitamin K reach different tissues in the body. This all begins with how they are incorporated into lipoproteins soon after we absorb them from food.
When we digest fat and fat-soluble nutrients, our intestines package them into lipoproteins known as chylomicrons, which take them through the lymph and into the bloodstream. This event critically distinguishes how water-soluble and fat-soluble nutrients are distributed through the body: water-soluble nutrients travel directly to the liver through the portal vein, while fat-soluble nutrients travel through the lymph in chylomicrons to bypass the liver and nourish the other tissues first.
Chylomicrons, like all other lipoproteins, have to transport fat-soluble things through the water-based environment of the blood. Therefore, they are fat-soluble on the inside and water-soluble on the outside.
While all K vitamins are fat-soluble, they are not all equally soluble in fat. Those with longer tails are more fat-soluble than those with shorter tails; for tails of equal length, saturated tails are more fat-soluble than unsaturated tails. K vitamins that are more fat-soluble are carried deeper in the core of chylomicrons, while those that are less fat-soluble are carried more toward the edges. Let’s take the three forms most commonly found in supplements as examples: K1, MK-4, and MK-7. We would expect to find MK-7 in the center of the chylomicron, MK-4 closer to the edges, and K1 in between the two (Schurgers and Vermeer, 2002).
Chylomicrons move in and out of the bloodstream rapidly, with a half-life of 15-20 minutes (César, 2006). This means that once we eat a meal, 95% of the chylomicrons that enter our blood are fully cleared in the first hour. Very few tissues actually take up the whole chylomicron. Instead, most tissues use the enzyme lipoprotein lipase (LPL) to siphon off its nutrients bit by bit. While LPL is best known for feeding the heart, skeletal muscle, and adipose tissue, it also feeds other tissues such as the lungs, kidneys, mammary glands, and brain (Kersten, 2014). LPL spreads across the capillary beds that feed our great diversity of tissues, allowing widespread access to the fat-soluble nutrients we ingest in a meal. Presumably, these tissues all have greater access to the nutrients carried closer to the edges of the chylomicrons, such as MK-4.
As these many tissues feast on the chylomicrons, the chylomicrons get smaller and smaller until they become chylomicron remnants. A small handful of tissues donate apolipoprotein E (ApoE) to the chylomicron remnants, and then use the LDL receptor and other related receptors to bind to the ApoE and take up the whole remnant. This allows them to score everything left in the particle right down to its chewy center. In this sense, ApoE is like the bait on a fishing line, and the receptor is like the hook. While the liver is best known for fishing out chylomicron remnants in this manner, our bones and spleen do as well. Our bones primarily derive nutrients through the uptake of whole lipoprotein particles, and take up about a fifth as many chylomicron remnants as our liver (Shearer, 2008). Thus, we should expect bone and liver to primarily have access to nutrients carried in the center of chylomicrons, including K1, but especially the MKs with longer tails, such as MK-7.
This whole stream of events takes place largely in the first hour after a meal. The liver then repackages the lipids it took in from chylomicron remnants into other lipoproteins, primarily VLDL, which are sent back out into the blood. Tissues continue to siphon off nutrients using LPL. Just like chylomicrons had been digested into chylomicron remnants, VLDL particles are then digested into LDL particles until our tissues take up the LDL particles themselves. Unlike the rapid clearance of chylomicrons, clearance of LDL particles takes place slowly over the course of two weeks (Langer, 1972). Although the liver is the main tissue that takes up LDL, bone is also important; in fact, bone takes up vitamin K more effectively from LDL than from any other lipoprotein (Shearer, 2012). Thus, K vitamins that get packaged into LDL particles will have a second opportunity to nourish bone.
Schurgers and Vermeer (2002) investigated how different K vitamins are transported using K1, MK-4, and MK-9. They fed six healthy males a mixture of one milligram of each form and took repeated blood measurements over four days, beginning at the two-hour mark. MK-4 had already peaked by the time the first blood draw was taken, when much of it was found in HDL, and disappeared most rapidly from the blood out of all the forms. K1 peaked at the four-hour mark, was mostly gone by eight hours, and disappeared by the end of the study. K1 was found almost exclusively in VLDL rather than in LDL or HDL. MK-9 peaked at the four-hour mark as well, but persisted in the blood for several days while carried in LDL particles.
The authors suggested that MK-4 was taken up so quickly because it was carried toward the edges of the chylomicrons, making it easily accessible for LPL-mediated extraction, with the excess spilling over into HDL particles. Notably, we should expect the extended circulation of MK-9 in LDL to provide better nourishment to bone.
Schurgers later collaborated with Sato (2012) to compare the bioavailability of MK-4 and MK-7 in healthy women. Compared to the 2002 study, they used less than half the dose of each vitamin and fed them separately rather than combined so that the total dose of vitamin K given at each point was over six times lower. Similar to the 2002 study, they took their first blood sample at two hours. They didn’t find MK-4 in the blood at any time point, whereas MK-7 remained elevated for two days.
MK-4 vs. MK-7: What Do We Really Know?
If we compare the results of the 2012 study to the earlier 2002 study, we can surmise that the dose of MK-4 in the 2012 study was low enough that the initial LPL feast in the first hour fully distributed it to a variety of tissues so that it was all gone by two hours, and that MK-7 circulated for such a long time because, like MK-9, it was redistributed in LDL particles. We should expect from this that MK-4 is good at nourishing most tissues, but not very good at nourishing liver or bone. By contrast, we should expect that MK-7 is good at nourishing the liver and even better at nourishing bone.
At the present time, there is no direct support for this, but there are hints that it may be the case. Sato (2012) cited a Japanese paper as finding that 1.5 milligrams of MK-4, but not 500 μg, improved the carboxylation of osteocalcin. Not even the abstract seems to be available in English, so it is difficult to evaluate the study. Later, Nakamura (2014) showed that only 600 μg of MK-4 is needed, but in this study the researchers simply gave the same people higher and higher doses each week and waited for osteocalcin carboxylation to improve. For all we know, their lowest dose, 300 μg, would have worked if they had given it longer than a week. In seeming contrast to MK-4, MK-7 improves osteocalcin carboxylation with as little as 100 μg (Knapen, 2012; Inaba, 2015).
Placing these studies side by side, they seem to suggest that improvements in osteocalcin carboxylation require much lower doses of MK-7 than of MK-4. However, the studies had different designs and were conducted in different populations that may have had different nutritional needs and different responses to vitamin K supplementation. In fact, Inaba (2015) fed MK-7 for four weeks while Nakamura (2014) only fed each dose of MK-4 for one week. This alone could explain the difference. To date, no one has compared the osteocalcin response to MK-4 and MK-7 head-to-head.
On the other hand, MK-7 has been compared to K1. At equal doses, MK-7 is three times more potent than K1 at carboxylating osteocalcin (Schurgers, 2007). Osteocalcin is made in bone, so its carboxylation reflects vitamin K status in that tissue. Presumably, MK-7 is better than K1 because its recirculation in LDL particles for days after it is first taken up by the liver gives it much more opportunity to nourish bone. Since MK-4 likely has even less opportunity to reach bone than K1, MK-7 is probably superior to MK-4 for this purpose as well.
What about other tissues? Unfortunately, we know even less about those. We know that large pharmacological doses of MK-4 given to rats (Konishi, 1973) or dogs (Sano, 1997) reach the lungs, liver, kidney, pancreas, spleen, adrenal gland, and bone very rapidly. Such large doses are also excreted into the feces in large amounts. More moderate nutritional doses could behave very differently, however, so it is difficult to form any conclusions from these studies. Until we have well designed trials comparing the ability of different MKs to support different health outcomes in humans, it makes sense to rely on what we know generally about how lipoproteins transport nutrients. This suggests K1 would best reach the liver, MKs 7-9 would best reach liver and bone, and MK-4 would best reach most other tissues.
MK-7 Supports Blood Clotting Better Than K1
MK-7 is not just three times better than K1 at reaching bone; it’s also five times better at supporting blood clotting (Schurgers, 2007). This may be because the greater fat-solubility of MK-7 makes it hold on more tightly to the membranes within liver cells, making it stay active in the liver much longer rather than being released and broken down (Shearer, 2008). The liver is where clotting proteins are made, so more extended activity in the liver would explain why MK-7 could better support blood clotting. If this is correct, other long-chain MKs such as MK-8 and MK-9 probably share this property as well.
MK-4 Plays a Unique Role in Gene Expression
MK-4 is unique among the K vitamins in its regulation of gene expression. It increases the expression of genes that regulate cell growth in osteoblasts (the cells responsible for bone growth), but MK-7 and K1 do not (Ichikawa, 2007). MK-4 increases testosterone production when fed to male rats. Cellular experiments show that MK-4, but not K1, increases testosterone by increasing the expression of the enzyme that converts cholesterol to pregnenolone, which is the first step in sex hormone synthesis (Ito, 2011).
MK-4 also inhibits the growth of various cancers of the liver, gut, and bone (Shearer, 2008). Remarkably, the gene that is now known to code for the enzyme that converts other K vitamins to MK-4, Ubiad1, was known years earlier as a tumor-suppressor gene (Shearer, 2014). Scientists observed that Ubiad1 was often silenced in tumors of the bladder, prostate, and kidney. Conversely, experimental overexpression of Ubiad1 inhibited the growth of prostate cancer cells. Since the enzyme that Ubiad1 codes for converts other K vitamins to MK-4, these results underscore that the anticancer properties of vitamin K belong specifically to MK-4.
Can We Rely on the Conversion of Other K Vitamins to MK-4?
When we consume any form of vitamin K, our intestinal cells break the side chains off of a small portion to yield the pure ring structure, known as menadione (Thijssen, 2006). Menadione then disperses through the body to many tissues that convert it to MK-4 for their own use by adding MK-4’s characteristic four-unit unsaturated side chain (Hirota, 2013).
We have known that animals synthesize MK-4 from other K vitamins for over a half century. It has been clear throughout that time, however, that the conversion varies widely. Early experiments, for example, showed that birds made the conversion better than rats and pigeons made it better than other birds (Billeter, 1960). Among rats, Wistar rats (Thijssen, 1994) seem to make the conversion better than Lewis rats (Ronden, 1998). Since the conversion varies between and within species, we should not assume that we as humans can make the conversion efficiently and consistently enough to meet our needs.
And just how good are we at this conversion? We really don’t know, but it stands to reason that it varies from person to person. Rare genetic defects in Ubiad1 have been identified (Yellore, 2007), and cancer is associated with epigenetic silencing of Ubiad1 (Woolston, 2015). Other genes involved in the conversion likely vary from person to person as well, but we don’t yet know what they are. One of them may be vitamin K epoxide oxidoreductase (VKOR), the target of warfarin. The normal role of VKOR is to reduce vitamin K that has been oxidized, and we know that menadione must be in a reduced state to undergo conversion to MK-4. Indeed, warfarin prevents the conversion of K1 to MK-4 in rats (Spronk, 2003). Genetic polymorphisms in VKOR are common (Shearer, 2012), and could hypothetically contribute to variation in MK-4 synthesis. We still do not know what enzyme is responsible for cleaving the side chain within our intestinal cells, and that could be polymorphic as well.
However good or bad humans may naturally be at the conversion, many people are taking medications that inhibit it (Hirota, 2015). Lipophilic statins such as lovastatin and simvastatin (and presumably atorvastatin, branded as Lipitor) inhibit the conversion. So do nitrogen-containing bisphosphonates such as alendronate (Fosamax) and zolendronate (Zometa), and presumably other nitrogenous bisphosphates as well. Ubiad1 expression depends on zinc (Funahashi, 2015) and its enzymatic activity depends on magnesium (Hirota, 2015), suggesting that deficiencies of either of these minerals could also compromise the conversion.
Finally, if we converted other K vitamins to MK-4 on a “however much we need to” basis, then it shouldn’t matter what type of vitamin K we consume at all. All forms of vitamin K generate some menadione in the intestine that can be converted to MK-4 in other tissues. Whether the menadione comes from K1, MK-4, MK-7, or any other form of vitamin K cannot make any difference in its tissue distribution. Humans accumulate MK-4 in multiple organs including the heart, lung, brain, liver, kidney, and pancreas (Thijssen, 1996). Thus, if there are no major limitations on the conversion besides our need for it, K1 should be perfectly capable of supplying these tissues with all the MK-4 they need, especially in populations that have high K1 intakes. Yet this does not seem to be what we find.
Consider the Dutch population, where this has been investigated most extensively. K1 intakes are eight times higher than K2 intakes, yet only K2 intake is inversely correlated with heart disease (Geleijnse, 2004; Gast, 2009; Buelens, 2009; Zwakenberg, 2016). In Germany, K1 intakes are about three times higher than K2 intakes, yet only K2 intake is inversely correlated with advanced prostate cancer (Nimptsch, 2008) and lung cancer (Nimptsch, 2010).
These observational studies don’t offer clear evidence of cause-and-effect relationships and they don’t show correlations with health endpoints that are specific to MK-4. However, they do add to the list of reasons to believe that our ability to synthesize MK-4 is limited by much more than our specific need for MK-4 itself, and by much more than our general need for vitamin K in the tissues that unconverted K1 has a hard time reaching. In other words, many of us probably need more MK-4 than we can make on our own, and that’s a good reason to eat foods that provide it.
Altogether, the evidence suggests that the form of vitamin K we consume matters, and that we are best served by a diversity of K vitamins from leafy greens, animal foods, and fermented foods.


For moms who come here looking for information on the vitamin K shot given to birth at babies, here's a great resource for a couple of unknown facts: https://substack.com/home/post/p-136535400
1) Polysorbate in Vitamin K injections opens up the baby's blood brain barrier, allowing more aluminum from the Hep B vaccines to get in their brains
2) One of the brands of Vitamin K injections contains benzyl alcohol, which can increase risk of jaundice.