The Biomechanics of Immune Dysfunction
This is the elephant in the autoimmunity and cancer room that EVERYONE IS IGNORING.
As I covered in my Crohn’s Protocol, and The Science Behind the Crohn’s Protocol, the big elephant in the room in Crohn’s is that biomechanical dysfunction from joint misalignments is probably the missing ingredient in Crohn’s that no one is paying attention to.
It follows from this that this might be broadly true of many diseases of immune dysfunction.
Most obviously, rheumatoid arthritis, where the immune dysfunction is probably directly precipitated by poor biomechanics in the joint.
This could, in fact, explain the study I first learned of from Andrew Huberman showing that ten minutes a day of stretching reduced breast cancer tumor volume in mice by 52%.
Despite an enormous body of research on the biochemistry and molecular biology of T cell activation, until recently there remained a longstanding unsolved puzzle: T cell activation requires an input of energy equivalent to two molecules of ATP, which is 40 times higher than what could be supplied by the ambient heat of body temperature, yet there was no biochemical reaction involved that could explain where the energy came from.
One observation that helped solve this puzzle was that T cells can never be activated in vitro when everything is dissolved in fluid. Rather, they can only be activated when the activation factors are immobilized on rigid surfaces such as beads or tissue plates.
In a living being, T cell activation occurs in the extracellular matrix, where T cells and the cells that present substances for them to mount a response to are anchored to cell surfaces or to proteins within the extracellular matrix.
It turns out that what provides the energy for T cell activation is mechanical force.
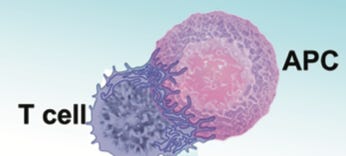
The substances that T cells decide to tolerate or mount a response to are called antigens. Antigen-presenting cells (APCs) present antigens to T cells to allow them to make this decision. The juncture between the APC and the T cell is called the immunological synapse:
The T cell connects to the APC with microvilli, tiny finger-like projections, that contain three major types of receptors: T cell receptors, costimulatory receptors, and coinhibitory receptors. Most antigens are peptides, which are small partially digested fragments of proteins. APCs filter them using major histocompatibility complex (MHC) proteins, which are genetically programmed filters that make some of us more likely to mount an immune response toward some things, and others toward others. The APC presents the antigen as a peptide-bound MHC protein (pMHC). Signaling molecules such as cytokines can encourage activation (costimulatory molecules, which bind to costimulatory receptors) or discourage activation (coinhibitory molecules, which bind to coinhibitory receptors).
The T cell also projects foot-like projections toward all the nearby cells and anything in the extracellular environment to detect changes in the structure and surface properties:
When a T cell receives a pMHC, successful activations of the T cell occur when there are many “catch bonds,” that facilitate stable bidirectional binding via the pMHC between the APC and the T cell. By contrast, peptides that do not activate the T cell tend to result in “slip bonds” where the T cell does not become stably joined to the APC via the pMHC:
The amount of mechanical force applied to the T cell helps select for how long these bonds last. Increasing force rapidly eliminates counter-productive slip bonds. An optimal amount of force, by contrast, dramatically lengthens the lifetime of the productive catch bonds, though excessive force eliminates them as well. Eliminating the slip bonds and maximizing the lifetime of the catch bonds maximizes the chances of activation. Therefore, a critical determinant of T cell activation is whether the optimal amount of mechanical force is applied.
There are, broadly speaking, two types of force that can be imposed on these connections: shear force, and push-pull force.
Shear force is parallel to the surfaces, while push-pull force is perpendicular to the surfaces.
During activation, “volleying” between tighter and looser connections has been observed. The tighter connection provides the force that activates a downstream signaling cascade, and the looser connection gives space for all the necessary components of that cascade to come together. By volleying between the two repeatedly, it allows repeated, sustained activation signaling.
This is coordinated by motor systems made from actin and myosin just like exists in contracting skeletal muscle (this is common in cells to use for internal contractile properties) that can facilitate the mechanical force involved:
However, the quality of the local environment is also critical to force generation:
Cancer cells, for example, evade the immune system by making their extracellular matrix overly rigid but making their cell surface overly soft. This maximally compromises local T cell activation.
Overactivity of the innate immune response in a respiratory infection can lead to fibrotic changes in the lungs that compromise T cell activation.
Liquid shear force has been shown to activate T cells in vitro, where it was suggested that high blood pressure could increase shear force and thereby drive autoimmunity; by contrast, it has been shown to dampen T cell activation when applied to periodontal ligament cells, which could imply that chewing would prevent gum inflammation, though fluid flow in the gums is probably also influenced by the mid infrared light generated during metabolism.
The researchers who study this are primarily interested in creating better vaccines and better immunotherapies for cancer.
I take this in a different direction.
The amount of force applied is likely a major determinant of the relative selectivity of the T cells for different antigens. If more force is applied (up to a certain point), the probability of activating is higher, which means that T cells that might otherwise not mount a response to a particular antigen may do so. Thus, selectivity would go down and the risk of autoimmunity would go up.
Most likely, optimal biomechanics and optimal fluid flow promote optimal activity of the immune system; poor biomechanics, inappropriately applied force, stagnancy, and hypertension promote immune dysfunction.
This highlights the importance of movement, metabolic activity, infrared light exposure, electrolyte balance, nervous system balance, and renal function in promoting optimal fluid flow.
It also highlights the importance of optimal biomechanics in the patterns of movement and alignments of the joints.
Joint fluid will not move the way it should when the joints are not moving the way they should. This could create immune dysfunction.
And if the joints are misaligned and putting inappropriate pressure on soft tissues, this could create immune dysfunction.
My take-home from this: we should all get a yearly checkup from a high-quality physical therapist or some other type of functional movement specialist to determine whether our joint alignment and movement patterns are optimized, and optimizing them should be an important part of our physical practice.
There are many reasons for this — see my article, Three Health Issues Converge on One Bone — for more examples, but an extremely under-appreciated reason may be that improper biomechanics are the elephant in the room we are ignoring in explaining many different types of immune dysfunction.








It makes a strong case for things like Taichuchuan, Baguazhang, etc. Thanks Chris.
You’re right and treatment/exercise can and should be highly specific not only to personal weak links (within the tensegrity system) but to the specific anatomy of the immune system. For example, the gastrosplenic ligament and splenorenal ligament, gerota fascia and zuckergandl fascia as it relates to spinal and rib position etc. etc. the vast majority of practitioners lack the assessment, treatment and analytical exercise tools to maximize effectiveness.