Melatonin Is Your Mitochondria's Guardian Angel
Installment two in our series on understanding the truth about SSRIs.
As our story moves on from the first installment in our journey toward understanding why Prozac is a performance-enhancing drug, why people who use SSRIs often develop sexual dysfunction, and why people who quit SSRIs can feel electric shocks running through their head as if they were trying to exit a relationship with an abusive partner with an electrifying temper, we need to properly define what serotonin is doing to our systems of energy metabolism.
Yet, we cannot do that without first acknowledging that it is the essential precursor to melatonin.
While melatonin has an essential role in syncing our sleep and wake cycle to the daily rhythm of light and darkness, it has a completely independent role inside our cells that is virtually unrelated to this rhythm and is primarily related to mitochondrial energy production.
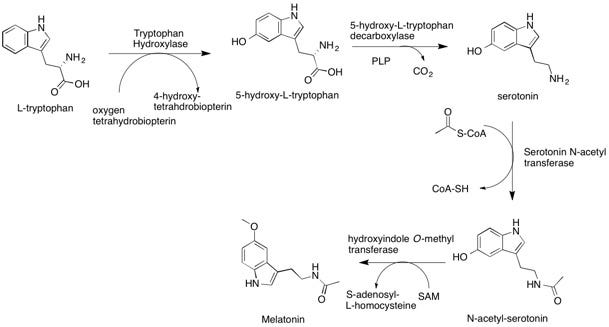
As shown below, serotonin is derived from the amino acid tryptophan, which is then acetylated by acetyl CoA and methylated by S-adenosylmethionine to produce melatonin.
The intracellular role of melatonin remains poorly understood and widely speculated upon, but the most informative study on the distribution of intracellular melatonin and its lack of relationship to the circadian rhythm is a rat study published in 2012.
Three groups of rats were studied. Two groups were subject to a 12-hour light, 12-hour dark cycle and either had their pineal gland removed or were subjected to a fake procedure that mimicked the surgery but did not remove the pineal gland. The third was exposed to light for 24 hours a day.
As expected, melatonin in the plasma remained low during exposure to light. It dramatically spiked after about 5 or 6 hours of darkness, largely returning to the normally low level by two hours before the lights came back on. In the rats that had their pineal gland removed, however, no spike in melatonin occurred. Thus, plasma melatonin is derived from the pineal gland in direct response to the light/dark cycle.
However, while the intracellular melatonin exhibits some variation across the day, it has no such pronounced relation to the light/dark cycle.
The circles below reflect the intracellular melatonin of normal rats and the triangles reflect rats who had their pineal gland removed.
Removing the pineal gland appears to initiate a stress response that increases intracellular melatonin synthesis.
This is the brain:
This is the liver:
Unfortunately, they did not report the diurnal variation of intracellular melatonin concentrations in rats exposed to continuous light.
However, they sacrificed rats at the 12-hour mark in all three groups:
The above figure shows the brain at the top and the liver on the bottom. Continuous light exposure tended to raise intracellular melatonin just like removing the pineal gland, though to a somewhat lesser degree.
This indicates that cells respond to the stress of circadian disruption by increasing their own synthesis of melatonin.
In the normal rats, however, it is highly likely that the circulating melatonin produced by the pineal gland is the primary source of cellular melatonin.
Why?
First, because, unlike serotonin, melatonin is fat-soluble enough to easily slip across cell membranes in a completely unregulated fashion. That is also why the melatonin seems to disproportionately accumulate in the membranes in this study.
Second, if you look at the diurnal variation, while there is no clear spike during the darkness, you can make out a general trend for melatonin to slip upwards during the dark and downwards during the light. My suspicion is the rapid disappearance of melatonin from plasma has almost nothing to do with its catabolism and almost everything to do with its cellular uptake. Inside cells, melatonin is not rapidly degraded. It largely sits there in watch.
In watch of what?
Well, the fact that intracellular melatonin synthesis flips into high gear during the stress of circadian disruption gives us a clue.
Removing the pineal gland from rats causes oxidative stress, oxidative damage, and inflammation in their eyes that is reversed by supplementing them with 5 milligrams per kilogram bodyweight melatonin. They thought this was from melatonin deficiency, but they didn’t measure the cellular melatonin. We know from the study above that it goes up. So circadian disruption puts stress on cells, they respond by increasing melatonin synthesis, but they don’t synthesize enough.
Rats an mice are nocturnal, yet their plasma and pineal melatonin behaves the same as that of humans’ in response to light. As reviewed here, even though bright light at night puts these rodents to sleep instead of keeping them up, it still disrupts their circadian rhythm and causes them to become obese and diabetic. Their daily rhythms of cortisol, insulin sensitivity, movement, and food intake become completely destroyed.
Data from rodents, non-human primates, and humans collectively suggest that there is a natural rhythmic ebb an flow of oxygen consumption that makes the mitochondrial respiratory chain slow down across the duration of the sleeping period and perk up when we are active. This requires adequate preparation for a number of reasons:
Pumping oxygen through blood actually requires considerable energy and during sleep all of our tissues are supposed to rest.
If influx of energy into the respiratory chain exceeds the availability of oxygen to finish metabolizing it, a “traffic jam” of electrons will ensue, creating potentially damaging reactive oxygen species.
Responding to this with post-hoc adaptive mechanisms also requires considerable energy and disrupts rest.
So it is likely that the energetic stress of circadian disruption is fundamentally a matter of failing to adequately prepare for the energetic shifts that are supposed to occur as we transition between sleeping and waking.
You can see in the above figures that melatonin is very enriched in brain mitochondria, and just moderately higher than the nucleus and cytosol in liver.
However, we will see in the next installment of this series that melatonin is synthesized in the mitochondria, consumed in the mitochondria, and leaves the mitochondria to act on the outside of the mitochondrial membrane in a way that fundamentally protects the mitochondria from stress, and that this protective effect goes far beyond the stress imposed by circadian disruption.
I hereby dub melatonin the “guardian angel” of the mitochondria because:
It is always present, keeping watch.
You may go by never noticing it or believing it is there.
When you need protection, that’s when it steps into action.
Read the next installment here:
Serotonin in the Mitochondria Means Your Cells Are Fighting to Survive
The next installment in our series on the true nature of SSRIs gets into the true nature of serotonin and its powerful impact on energy metabolism.









Great post, with the thorough elaboration you give every metabolic issue! I love
«I hereby dub melatonin the “guardian angel” of the mitochondria because:
• It is always present, keeping watch.
• You may go by never noticing it or believing it is there.
• When you need protection, that’s when it steps into action.»
interesting how melatonin synthesis ramps up in response to circadian stress. wouldn’t that suggest its presence is less a sign of optimal health and more a signal that the cell is in defensive mode? in which case, chronically elevated melatonin might not indicate restfulness but energetic insufficiency?